In vitro production of cat-restricted Toxoplasma pre-sexual stages - Nature.com
Abstract
Sexual reproduction of Toxoplasma gondii, confined to the felid gut, remains largely uncharted owing to ethical concerns regarding the use of cats as model organisms. Chromatin modifiers dictate the developmental fate of the parasite during its multistage life cycle, but their targeting to stage-specific cistromes is poorly described1,2. Here we found that the transcription factors AP2XII-1 and AP2XI-2 operate during the tachyzoite stage, a hallmark of acute toxoplasmosis, to silence genes necessary for merozoites, a developmental stage critical for subsequent sexual commitment and transmission to the next host, including humans. Their conditional and simultaneous depletion leads to a marked change in the transcriptional program, promoting a full transition from tachyzoites to merozoites. These in vitro-cultured pre-gametes have unique protein markers and undergo typical asexual endopolygenic division cycles. In tachyzoites, AP2XII-1 and AP2XI-2 bind DNA as heterodimers at merozoite promoters and recruit MORC and HDAC3 (ref. 1), thereby limiting chromatin accessibility and transcription. Consequently, the commitment to merogony stems from a profound epigenetic rewiring orchestrated by AP2XII-1 and AP2XI-2. Successful production of merozoites in vitro paves the way for future studies on Toxoplasma sexual development without the need for cat infections and holds promise for the development of therapies to prevent parasite transmission.
Similar content being viewed by others

Autonomous transposons tune their sequences to ensure somatic suppression
Mitochondrial DNA replication stress triggers a pro-inflammatory endosomal pathway of nucleoid disposal
N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting
Main
Toxoplasma, the cause of the global zoonotic infection toxoplasmosis, presents a multifaceted life cycle with distinctive stages (Fig. 1a). Much is understood about its fast-growing tachyzoites and semi-dormant bradyzoites, but its sexual reproduction, confined to felid guts, is less explored. Each stage has a distinctive transcriptional signature and switching between them is regulated by intricate transcriptional cascades in which covalent and noncovalent epigenetic mechanisms act as driving forces3,4. The chromatin modifiers MORC and HDAC3, key players in gene silencing1,2, act as critical checkpoints for sexual commitment, and when conditionally depleted or inhibited in tachyzoites, they trigger broad activation of chronic and sexual gene expression1,2. Previous studies overlooked their combined presence in nucleosomal telomeric repeats1, suggesting a secondary role in maintaining genome stability through the formation of telomeric heterochromatin (Extended Data Fig. 1a). MORC detachment from chromosome ends disrupts subtelomeric gene silencing (Extended Data Fig. 1b), causing telomere dysfunction, mitotic bypass and aberrant polyploid zoite accumulation (Extended Data Fig. 1c), reminiscent of aneuploidy in human cancer5. In MORC-depleted parasites, disorganized telomeres may in turn disrupt gene-level transcriptional regulation, leading to misguided sexual development. An alternative way to explore the modus operandi of MORC involves its partners, the apetala proteins1 (AP2; Extended Data Fig. 2a), which are considered important regulators of life-cycle transitions in all apicomplexan species3,6. This study underscores the role of two AP2 repressors in coordinating the expression of stage-specific genetic programs, and thus controlling Toxoplasma merogony.
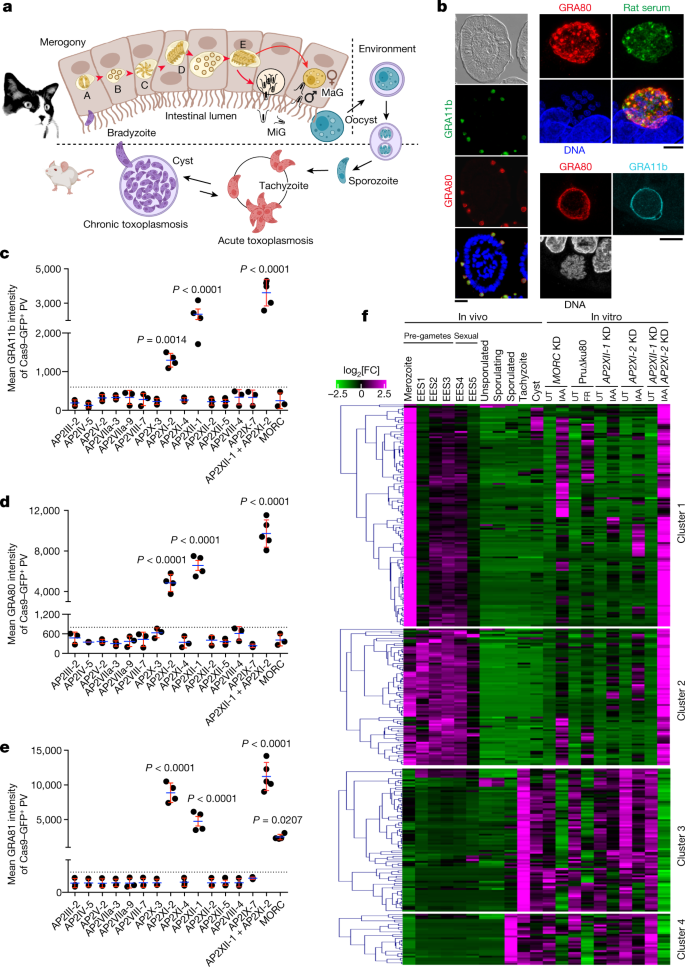
a, T. gondii has a multistage life cycle. The enteroepithelial cycle begins when a cat ingests tissue cysts, initiating asexual replication of merozoites (morphotypes A–E) leading to production of macro-gametes (MaG) and micro-gametes (MiG). These form oocysts that sporulate in an oxygen-rich environment. After ingestion, released sporozoites develop into tachyzoites causing acute infection. Later, owing to host immunity, they convert into bradyzoites forming tissue cysts. Created with BioRender.com. b, IFA images of infected cat intestine using GRA80 and GRA11b antibodies and rat serum; nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 25 μm (left column of images), 2 μm (top right four images) and 5 μm (bottom right three images). c–e, Expression of the merozoite markers GRA11b (TGME49_237800; c), GRA80 (TGME49_273980; d) and GRA81 (TGME49_243940; e) was quantified in tachyzoites in which MORC, 1 of 14 MORC-associated AP2 proteins or AP2XII-1 and AP2XI-2 were genetically disrupted. Cas9–GFP measures genetic disruption efficacy (Extended Data Fig. 2f). Horizontal bars represent mean ± s.d. of vacuolar proteins intensity from n = 3 (c,d) and n = 4 (e) independent experiments (n = 50 GFP+ vacuoles per dot). The P values were determined using one-way analysis of variance (ANOVA) and Tukey's test. PV, parasitophorous vacuole. f, Differential expression analysis was carried out with DESeq2 on raw rRNA-subtracted data, with Benjamini–Hochberg correction (P value threshold of 0.05). In the IAA (24 h)-induced double-KD parasites, 295 genes exhibited upregulation (log2[FC] > 2) and 195 genes exhibited downregulation (log2[FC] < −1), compared with untreated (UT) parasites. The heat map uses mean-centred data and k-means clustering (Pearson correlation) using iDEP.96, and shows log2-transformed data. Hierarchical clustering grouped genes and samples to elucidate expression patterns across different in vivo stages—merozoites, enteroepithelial stages (EESs) 1–5, tachyzoites, sporozoites and cysts—as documented in previous studies12,13,16. We also examined these patterns in the context of in vitro MORC KD and HDAC3 inhibition with FR235222 (FR; ref. 1). Transcript abundance is shown as log2[FC] based on the log-transformed mean transcripts per million kilobases (TPM) values from three biological replicates. Magenta indicates upregulation, and green indicates downregulation.
Source Data
AP2-mediated merozoite gene silencing
In the 1970s, Toxoplasma's sexual cycle was partially studied in infected kittens through meticulous examination of the ultrastructure of the pre-gametes zoites and sexual dimorphic stages in the intestinal lining of Felis catus7,8,9,10,11. Merozoites—the initiators of the sexual cycle—have a unique transcriptional profile12,13, but their study has been difficult owing to the lack of specific markers. The only recognized marker so far, GRA11b, is used to track the development of this stage in the gut of Toxoplasma-infected cats14 (Fig. 1b). For a more in-depth exploration of merogony, we identified three potential merozoite-specific proteins with gene expression profiles mirroring those of GRA11b and produced matching antibodies. Notably, antibodies to TGME49_273980 showed robust reactivity with feline merozoites (Fig. 1b), but not with tachyzoites or bradyzoites, whether converted in vitro by overexpressing BFD1 (ref. 15) or present in tissue cysts in mouse brain (Extended Data Fig. 2b,c). This protein, together with GRA11b, is localized in the vacuolar space and on parasitophorous vacuole membranes, and has the typical characteristics of a dense granule protein; it is hereafter referred to as GRA80 (Fig. 1b). We were unable to produce antibodies compatible with immunofluorescence assay (IFA) for TGME49_243940, but the epitope-tagged protein shows typical features of a dense granule protein (GRA81) that accumulates in the vacuolar space after MORC depletion1 (Extended Data Fig. 2d). Another protein, GRA82 (TGME49_277230), co-occurs with GRA11b in in vivo schizonts specifically (Extended Data Figs. 2e and 3d).
Using these new markers, we investigated which MORC- and HDAC3-associated AP2 is responsible for repressing merozoite-specific gene expression in tachyzoites. In a CRISPR loss-of-function screen, inactivation of only AP2XI-2 or AP2XII-1 among the 14 MORC-associated AP2 proteins resulted in a significant increase in the expression level of GRA11b, GRA80 and GRA81–HA to varying degrees (Fig. 1c–e and Extended Data Fig. 2f). This suggests a common or overlapping role of these two transcription factors. To examine the genetic relationship between AP2XI-2 and AP2XII-1, we knocked out both genes simultaneously and observed a synergistic increase in the expression level of all three merozoite markers that exceeded threefold the levels observed in the individual knockouts (Fig. 1c–e). By contrast, MORC knockout led to only a low level of expression of GRA81–HA (Fig. 1e). AP2XI-2 and AP2XII-1 are essential for tachyzoite proliferation (Extended Data Fig. 2a), hindering the study of merogony with knockouts. To investigate this further, we used the minimal auxin-inducible degron system (mAID; Supplementary Table 1) and transiently knocked down each AP2 factor individually or simultaneously (Extended Data Fig. 2g). Single knockdowns (KDs) of AP2XI-2 and AP2XII-1 had limited effects on merozoite marker expression, as less than 25% of vacuoles were co-labelled with GRA11b and GRA80 (Extended Data Fig. 3b). However, simultaneous KD resulted in efficient merozoite differentiation with more than 98% of vacuoles showing both markers after 48 h (Extended Data Fig. 3a,b). By contrast, MORC-depleted vacuoles did not exhibit a significant level of coexpression of GRA11b and GRA80 (Extended Data Fig. 3b). Additionally, KD of three genes encoding other MORC-associated AP2 proteins (AP2VII-3a, AP2VIII-4 and AP2VIII-7) failed to induce merozoite marker expression (Extended Data Fig. 3c).
Notably, GRA82 showed delayed but significant coexpression with GRA11b in 98% of vacuoles depleted of both AP2XII-1 and AP2XI-2, 48 h after 3-indoleacetic acid (IAA) addition. This suggests a potential temporal regulation or association with a specific merozoite morphotype (Extended Data Fig. 3d,e). The in vitro pre-sexual parasite population that emerged following the acute depletion of AP2XI-2 and AP2XII-1 is homogeneous and does not express the typical markers of tachyzoites (for example, GRA2; Extended Data Fig. 3f,g) and bradyzoites (for example, BCLA (ref. 1), BAG1 and DBA; Extended Data Fig. 3h–j). In comparison, accumulation of Shield-protected BFD1 (ref. 15) induced bradyzoite differentiation in more than 95% of parasites (Extended Data Fig. 3h–j), without the expression of merozoite markers (Extended Data Figs. 2b and 3b). In contrast to the AP2 double-KD mutant, MORC-depleted parasites showed asynchronous development1 with vacuoles expressing either bradyzoite (BCLA+) or merozoite (GRA81+) markers in a mutually exclusive manner (Extended Data Fig. 3k).
To gain a more complete understanding of in vitro merozoite differentiation beyond the limited perspective offered by GRA11b and GRA80 co-staining, we carried out RNA sequencing (RNA-seq) on all KD strains to investigate genome-wide transcriptional changes resulting from individual or simultaneous depletion of AP2XI-2 and AP2XII-1. Analysing the RNA-seq data using DESeq2, we identified 490 differentially expressed transcripts (fold change (FC) threshold of ≥8 and P value < 0.05), including 295 upregulated genes and 195 downregulated genes when both AP2XI-2 and AP2XII-1 were depleted (Fig. 1f and Supplementary Table 2).
Hierarchical clustering analysis revealed that the co-depletion of AP2XI-2 and AP2XII-1 resulted in gene expression profiles reminiscent of those observed in vivo in enteroepithelial stages (EESs)12,13,16. Their acute degradation triggered the induction of pre-gamete-specific genes at different developmental stages (clusters 1 and 2), while concurrently repressing a subset of tachyzoite-specific genes (clusters 3 and 4; Fig. 1f). These transcriptional changes mirror the gene expression profile observed in merozoites in the cat intestine12,13. Using principal component analysis, we observed consistent clustering of biological replicates within each treatment, indicating excellent reproducibility. In addition, the samples showed substantial clustering based on genetic background with significant separation between single-KD and double-KD samples (PC1 = 58%), suggesting synergistic regulation of gene expression by AP2XI-2 and AP2XII-1 in Toxoplasma (Extended Data Fig. 4a). DESeq2 analysis revealed that depletion of both AP2 factors was found to be necessary to upregulate 65% (194/295) of the identified genes (FC ≥ 8; P value < 0.05; Extended Data Fig. 4b). By contrast, single KDs of AP2XI-2 and AP2XII-1 resulted in expression of a lower proportion of genes, 9% and 13.5%, respectively (Extended Data Fig. 4b). This transcriptional trend also extends to tachyzoite-specific genes, whose repression is quantitatively more pronounced when AP2 proteins are simultaneously depleted (Extended Data Fig. 4c).
Changes in mRNA levels in response to IAA treatment translated into changes in protein abundance. Principal component analysis showed consistent clustering of biological replicates within each condition, indicating high reproducibility (Extended Data Fig. 4d). Proteomic analysis revealed robust changes in 18% of the parasite proteins (n = 3,020 detected; log2[FC] ≥ 1; P value ≤ 0.01), with a highly polarized response to the merozoite stage. IAA-treated parasites exhibited increased expression levels of 276 proteins associated with pre-gamete stages, whereas 285 tachyzoite proteins were suppressed (Extended Data Fig. 4e and Supplementary Table 3). Overall, the RNA and protein expression patterns of in vitro merozoites mirrored those observed in their enteroepithelial counterparts12,13.
AP2 depletion causes stage conversion
The process of invasion of Toxoplasma tachyzoites has been thoroughly examined, revealing the cryptic functions of organelle-resident proteins17. Micronemes secrete adhesin proteins (MICs) for attachment, facilitating motility and invasion. Rhoptries release neck (RON) and bulb (ROP) proteins, which interact with MICs to breach the host cell membrane and form the parasitophorous vacuole. Dense granules (GRA) release proteins involved in intravacuolar function, such as the tubulovesicular network, and act as effectors at the parasitophorous vacuole membrane and beyond, manipulating host signalling18. Most MIC, ROP and GRA proteins secreted by tachyzoites and bradyzoites are absent in merozoites isolated from cats12,13,19,20. In vitro, we also observed substantial changes in the expression of many known MIC, ROP and GRA proteins after the addition of IAA, providing evidence for a developmental switch (Fig. 2a–c and Supplementary Table 2). Specifically, MIC complexes that play important roles in tachyzoites (for example, MIC2 and AMA1) were repressed, whereas merozoite- and bradyzoite-specific MICs (for example, MIC17a, MIC17b, MIC17c and AMA2) were markedly induced. Notably, the reassortment of organelle-resident proteins seems to be highly specific, as MICs restricted to sporozoites (SporoAMA1) were not induced in response to IAA (Fig. 2a).
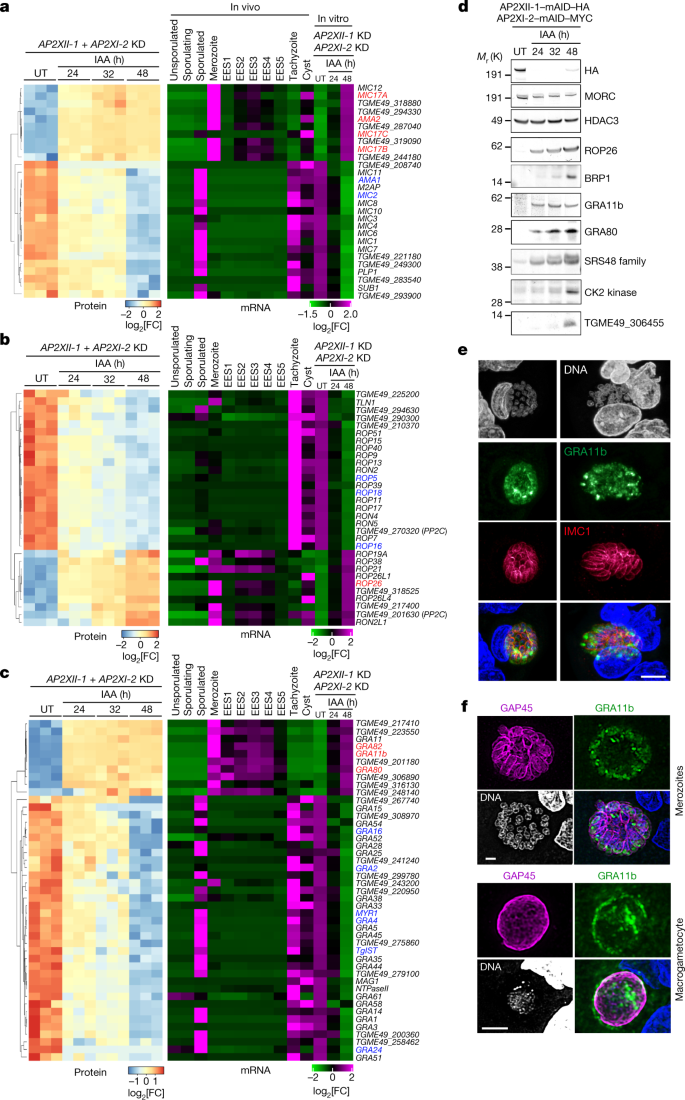
a–c, Heat map showing hierarchical clustering analysis of selected microneme (a), rhoptry (b) and dense granule (c) mRNA transcripts and their corresponding proteins, which were significantly upregulated (log2[FC] > 2; P value < 0.05) or downregulated (log2[FC] < −1; P value < 0.05) following the simultaneous depletion of AP2XII-1 and AP2XI-2. The abundance of these transcripts is presented across different in vivo stages—merozoites, EES1–EES5 stages, tachyzoites, sporozoites and cysts—as documented in previous studies12,13,16. Pertinent examples of tachyzoite and merozoite stage genes are highlighted in blue and red, respectively. Analysis parameters are those of Fig. 1f. d, Time-course western blot analysis of protein expression levels after depletion of AP2XII-1–mAID–HA and AP2XI-2–mAID–MYC. Samples were collected at the indicated time points after addition of IAA and probed with antibodies to HA, MORC, HDAC3, rhoptry proteins (ROP26 and BRP1), dense granule proteins (GRA11b and GRA80), the SRS48 family, a CK2 kinase (encoded by TGME49_307640) and the protein encoded by TGME49_306455. The experiment was repeated three times and a representative blot is shown. e,f, Maximum-intensity projection of a confocal microscopy z-stack from a meront in infected small intestine of a kitten. Antibodies to GRA11b (green) mark the dense granules and those to IMC1 (red) or GAP45 (magenta) mark the IMC of individual merozoites. Nuclei were counterstained with DAPI. Scale bars, 5 μm (e) and 2 μm (f).
Source Data
The combined depletion of AP2XI-2 and AP2XII-1 silenced 80% of the 143 rhoptry proteins described or predicted by hyperLOPIT to be tachyzoite specific (Fig. 2b). These include the components of the tachyzoite complex of RON2, RON4, RON5 and RON8 as well as ROP16, ROP18 and ROP5, which function as effectors to protect parasites from host cell-autonomous immune defences18. The number of ROP proteins reported to be exclusively specific to pre-gametes is rather limited (Fig. 2b). Among them, BRP1 stands out as the first protein described to be expressed in both merozoites and bradyzoites21 (Fig. 2d). ROP26 is also expressed in both in vitro merozoites and BFD1-expressing bradyzoites (Fig. 2b,d and Extended Data Fig. 4f). These proteins, present in both stages, may facilitate the transition process from bradyzoites to merozoites.
When we examined GRA mRNA and protein levels in response to IAA treatment, we observed extensive and comparable shutdown of the tachyzoite program and activation of the merozoite program (Fig. 2c). Notably, the levels of core proteins of the tubulovesicular network, including the GRA2 archetype, declined significantly in in vitro merozoites over time (Extended Data Fig. 3f,g). The MYR-dependent effectors GRA16, GRA24 or TgIST also showed a comparable decrease (Fig. 2c). By contrast, confirmed GRA markers of pre-gametes (for example, GRA11b (ref. 14), GRA80, GRA81 and GRA82) were induced in response to IAA (Fig. 2c,d and Extended Data Fig. 3a,b,d,e).
During the transition from tachyzoite to merozoite, surface proteins on the zoite also undergo significant restructuring, including the SAG-related surface (SRS) protein family12,13. Compared to tachyzoites, merozoites express a broader range of SRS proteins (Extended Data Fig. 4g)—for example, SRS48 (Fig. 2d) and SRS59—which may contribute to gamete development and fertilization12,13. IAA treatment induces the expression of 90% of the known 88 SRS proteins, effectively mimicking the phenotypic features of in vivo merozoites, whereas suppressing tachyzoite-specific SRS proteins (Extended Data Fig. 4g). In addition, 29 of the 33 family A members, representing the major membrane-associated merozoite proteins, are expressed in vitro during this transition to pre-gametes (Extended Data Fig. 4h).
In vitro-produced merozoites are deficient in essential proteins necessary for tachyzoite functions, including motility, attachment and invasion. As a result, these zoites exhibited reduced infectivity in human fibroblasts, as indicated by a notable decrease in the number of lytic plaques compared to that of untreated parasites (Extended Data Fig. 5a).
AP2-depleted zoites undergo merogony
In 1972, Dubey and Frenkel characterized pre-sexual stages at the cellular level and identified five morphological stages (A–E) that form sequentially during colonization of the cat intestinal epithelium before gamete formation22 (Fig. 1a). These morphotypes can be differentiated on the basis of their distinct subcellular structures and nuclear content23. Using transmission electron microscopy and immunofluorescence, we tracked the development of these stages during in vitro merogony, including the dynamic behaviour of the inner membrane complex (IMC), a unique organelle essential for daughter cell formation during replication. During development in the cat intestine, the functions of various IMC proteins diverge from their assigned roles in tachyzoites23. For example, at the later stages of schizogony, IMC1 and IMC3 staining revealed daughter IMC, whereas IMC7 staining was restricted to the periphery of the mother cell23. In agreement with these findings, our results show that IMC1 but also GAP45 are valuable markers for tracking merozoite division during their development in the intestinal mucosa of cats (Fig. 2e,f).
As a first step, 24 h post-IAA addition, the nucleus of the mother cell undergoes several fission events while maintaining the nuclear envelope, leading to individualized nuclei (in even numbers, ranging from 4 up to 10; Fig. 3a). Concomitantly, single organelles such as the apicoplast and the Golgi apparatus expand and multiply to match the number of nuclei. Transversal cross-sections of the apicoplast (limited by four membranes) reveal its elongation and constriction, suggestive of replication by scission (Fig. 3b), which was also visualized by IFA with the ATrx1 antibody24 (Extended Data Fig. 5b) and is in line with maternal inheritance of the apicoplast seen in meronts in the cat intestine25. Multiple Golgi complexes are formed at different sites of the nucleus, sometimes in opposing orientations, suggestive of de novo formation (Fig. 3c). The manner in which other organelles, such as secretory organelles, multiply is not yet known. At this stage, the multinucleated mother cell contains several sets of organelles randomly distributed throughout the cytoplasm. Despite the increase in size of the mother cell, the subpellicular IMC is still prominently present beneath the plasma membrane. The parasites exhibiting a characteristic ovoid shape with four and eight nuclei are morphologically related to the cryptic and early meronts, namely B and C morphotypes22 (Fig. 3d and Extended Data Fig. 5c).
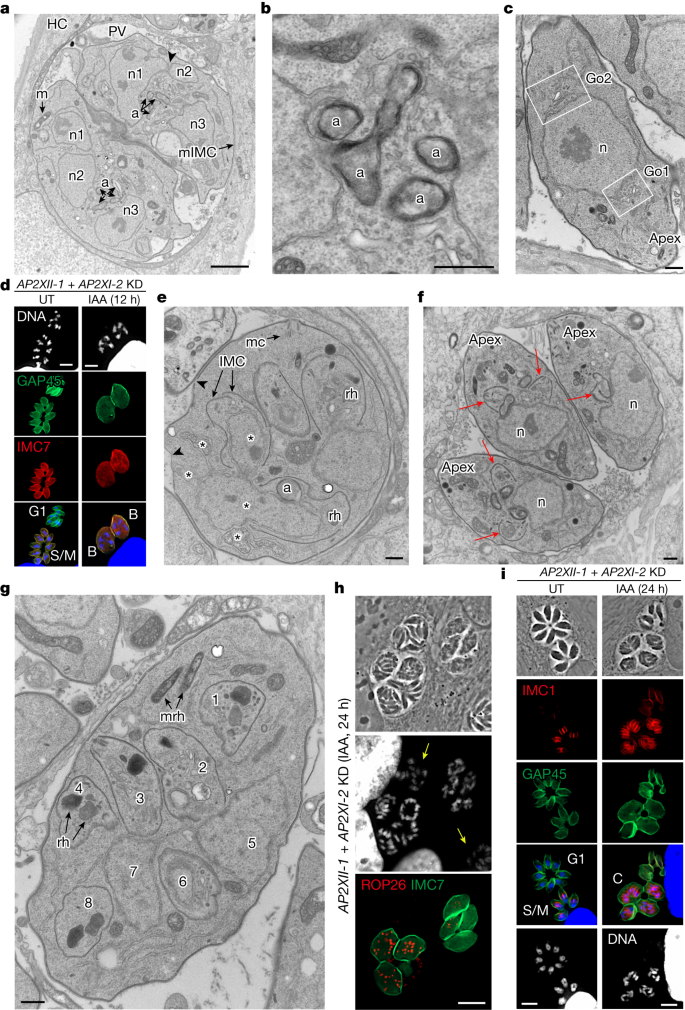
a–c,e–g, Electron micrographs of human foreskin fibroblast (HFF) cells infected with the RH (AP2XII-1 and AP2XI-2 KD) strain of T. gondii, untreated (f) or treated for 24 h (a–c,e,g) with IAA. a, Emphasis on karyokinesis with fission. n1 to n3, nuclear profiles; a, apicoplast; m, mitochondrion; mIMC, mother IMC; HC, host cell. The arrowhead highlights an area of nuclear fission. b, Emphasis on apicoplast multiplication by growth and scission. c, Emphasis on Golgi multiplication from either side of the nucleus (n). Go1 and Go2, two Golgi apparatus. d, IFA of tachyzoites (untreated) and zoites depleted of AP2XII-1 and AP2XI-2 (12 h post-IAA). GAP45 staining marks the mother cell and its progeny. IMC7 staining specifically marks the diploid (left) and polyploid (right) mother cell. Cells were stained with Hoechst DNA-specific dye. Type B meronts are marked in yellow. e, Emphasis on appearance and role of the IMC segregating daughter buds in the mother cytoplasm. mc, mother conoid; rh, rhoptry. The arrowheads show areas devoid of the IMC and the asterisks highlight areas of nuclear fission. f, Emphasis on contrasting endodyogeny in tachyzoites (untreated condition). Two daughter buds formed apically and symmetrically (arrows). g, Emphasis on endopolygeny showing up to eight daughter buds and ultrastructure of rhoptries. mrh, mother rhoptry. h,i, Tachyzoites (untreated) and merozoites depleted of AP2XII-1 and AP2XI-2 (24 h post-IAA) were fixed and stained for ROP26 (TGME49_209985, in red) and IMC7 (green; h) or IMC1 (red) and GAP45 (green) along with Hoechst DNA-specific dye (i). Yellow arrows indicate IMC7− mature merozoites, and type C meronts are shown. Scale bars, 2 μm (a), 500 nm (b,c,e–g), 5 μm (d,i) and 10 μm (h). G1, cell growth phase before DNA synthesis; S/M, DNA synthesis (S phase) and mitosis (M phase).
As a second step, new flattened vesicles of the IMC emerge in the mother cytoplasm, and progressively elongate allowing the sub-compartmentalization of organelles destined for each daughter cell (Fig. 3e). This process of internal budding of more than two daughter cells, referred to here as endopolygeny26,27,28,29,30, differs from the tachyzoite division by endodyogeny in which the two daughter cells are generated symmetrically and in a synchronous manner in the mother cell (Fig. 3f). Alongside the expansion of daughter buds, the mother IMC and conoid undergo partial disassembly. Notably, rhoptries inside daughter cells are different in shape and electron density from mother rhoptries dispersed in the cytoplasm, suggesting de novo biogenesis of rhoptries (Fig. 3g), which can also be traced with ROP26 (Fig. 3h). This finding aligns with the observation that the bulbous end of the rhoptry in in vivo meronts remains spherical, in contrast to that in tachyzoites and bradyzoites31.
In these multinucleated structures, daughter IMC could be identified with IMC1 staining, whereas GAP45 staining was restricted to the periphery of the mother cell (Fig. 3i and Extended Data Fig. 5d). Daughter cells become polarized with the formation of a conoid and apical distribution of micronemes, rhoptries, the apicoplast, and the Golgi apparatus (Fig. 4a and Extended Data Fig. 5e). At this stage, it is evident that the maternal conoid coexists with the newly formed conoids of the progeny, as shown by the labelling of apically methylated proteins (Extended Data Fig. 5f). After final assembly, the daughter cells emerge separately, wrapped by the plasma membrane of the mother cell (cortical or peripheral budding; Fig. 4b,c), forming fan-like structures as previously described in infected cat cells26. Compared to tachyzoites, these newly formed parasites are thinner and do not form a rosette-like structure within the parasitophorous vacuole but instead are aligned, with their apex facing the parasitophorous vacuole membrane (Extended Data Fig. 5g,h); these features are reminiscent of those of type-D-like merozoites arising in cat intestinal cells from meront entities at the onset of infection28,29,30. Notably, the parasitophorous vacuole membrane of merozoites also forms physical interactions with host ER and mitochondria, potentially for nutrient acquisition32 (Extended Data Fig. 5h).
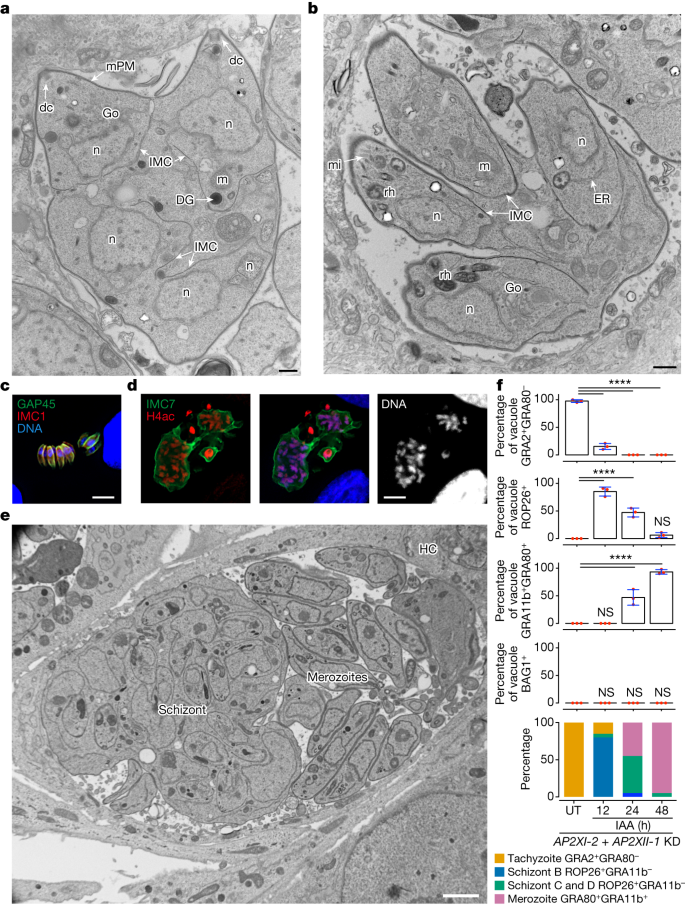
a,b,e, Electron micrographs of RH (AP2XII-1 and AP2XI-2 KD)-infected HFFs treated for 24 h (a,b) or 48 h (e) with IAA. a, Emphasis on protruding daughters sharing the mother plasma membrane (mPM). DG, dense granule; dc, daughter conoid. b, Emphasis on daughter cell emergence. mi, microneme; ER, endoplasmic reticulum. c, Representative image of neatly aligned elongated merozoites and forming fan-like structures as they hatch from the mother cell. Mature merozoites are co-stained with GAP45 (green), IMC1 (red) and Hoechst DNA-specific dye (blue). d, Image of a giant schizont delineated by IMC7 (green) showing polyploidy (n = 16). The nuclear structure is co-stained with Hoechst DNA-specific dye and hyperacetylated histone H4 (H4ac; red). e, Emphasis on a large parasitophorous vacuole containing a mega meront with many daughter buds residing with merozoites in the same parasitophorous vacuole. f, Time-course analysis of marker expression following AP2XII-1 and AP2XI-2 co-depletion. Data represent mean ± s.d. of vacuole staining for GRA2+GRA80−, ROP26+, GRA11b+GRA80+ or BAG1+ from three experiments (n = 50 vacuoles per dot). Statistical evaluation was conducted using one-way ANOVA, followed by Tukey's multiple comparison test. Graph at bottom: longitudinal tracking of tachyzoites and developmental morphotypes within vacuoles post-IAA treatment, integrating stage-specific aforementioned markers and parasite nuclei count (Hoechst and histone staining). Scale bars, 500 nm (a,b), 5 μm (c), 10 μm (d) and 2 μm (e).
In vitro merozoites mimic cat pre-gametes
Extending the IAA treatment for another 16 h reveals the presence of very large meronts containing numerous daughter cells in formation (Fig. 4d,e). These schizonts are detected in the same parasitophorous vacuole together with fully formed in vitro merozoites (Fig. 4e), the latter being very similar to their counterpart in the cat intestine (Fig. 2e,f). Mature polyploid meronts can be visualized by IMC7 staining on their surface, whereas fully formed merozoites were completely negative for IMC7 (Fig. 3d,h and Extended Data Fig. 5c), a phenotype that has also been observed in pre-gametes developing in the cat gut23. Notably, new pre-merozoite or merozoite-specific markers such as ROP26 and GRA80, respectively, distinguish the two morphotypic populations. ROP26 marks zoites undergoing schizogonic replication, whereas GRA11b and GRA80 are expressed exclusively in mature in vitro merozoites (Fig. 3h and Extended Data Fig. 6a). As merozoites undergo several cycles of endopolygeny, they acquire new distinct morphological features compared to first-generation merozoites (24 h post-IAA), probably type E (ref. 26). Some in vitro merozoites are sausage shaped, with a diameter of 1.5–1.8 μm, packed in the parasitophorous vacuole without any spatial organization (Extended Data Fig. 6b,c). These forms contain similar organelles found in tachyzoites, but they exhibit an extruded conoid (Extended Data Fig. 6d). Other parasitophorous vacuoles contain peripherally arranged parasites, leaving a large empty space (Extended Data Fig. 6e,f), reminiscent of schizont parasitophorous vacuoles formed in cat intestinal cells26. Notably, these parasites at the parasitophorous vacuole edge adopt two configurations: a very large cell body (trapezoid) with a diameter of up to 5 μm or a very thin and elongated shape (tubular) with a diameter of 200–250 nm (Extended Data Fig. 6g,h). The latter do not contain nuclei but mitochondrion profiles and ribosomes are observed. Their origin and formation remain to be determined but their abundance in parasitophorous vacuoles probably suggests a physiological relevance in the Toxoplasma life cycle.
Morphotypes A–E are difficult to study in vivo because they vary in size and shape and develop asynchronously in different regions of the digestive tract19,20,26,31,33. Here we were able to follow the initial steps of in vitro merogony using several stage-specific markers. Asynchronous nuclear division cycles were detected by DNA, histone or centrosome staining (Extended Data Fig. 7a–d). At 12-h post-IAA treatment, a significant proportion of the tachyzoite population transitioned into morphotype B (3–4 nuclei and ROP26+), reaching up to 75% (Fig. 4f). Within 24 h, morphotypes C and D (8–32 nuclei and ROP26+) and mononuclear merozoites (GRA11b+GRA80+) coexisted in culture (Fig. 4f). After 48 h, nearly 98% of the parasite population expressed merozoite markers (GRA11b+ and GRA80+), whereas typical tachyzoite (GRA2) or bradyzoite (BAG1) markers were absent (Fig. 4f), aligning with our extensive transcriptome and proteome analyses.
AP2 proteins bind to MORC and HDAC3
AP2XI-2 and AP2XII-1 probably synergize to suppress gene expression in tachyzoites, but their modus operandi is still enigmatic. Both proteins were originally found in a MORC pulldown along with HDAC3 in tachyzoites1. We confirmed their strong and specific association with MORC and HDAC3 by reverse immunoprecipitation combined with mass spectrometry (MS)-based quantitative proteomic and western blot analyses using knock-in parasite lines expressing a Flag-tagged version of AP2XI-2 or AP2XII-1 (Fig. 5a,b). Each AP2 protein shows significant enrichment in the eluate of its corresponding counterpart (FC > 90; P value < 1.5 × 10−10), indicating their association within the same functional complex along with MORC and HDAC3 (Fig. 5a). Notably, this interaction is specific and exclusive to these two AP2 proteins, as no such association was observed with other AP2 proteins, or with other chromatin modifiers (Supplementary Table 4). HDAC3 and MORC exhibited comparatively lower levels of enrichment (FC of 8 and 14, respectively, Fig. 5a), suggesting that AP2XI-2 or AP2XII-1 proteins independently form heterodimers in cellular contexts. To further test this hypothesis, we used baculoviruses to transiently coexpress epitope-tagged AP2XII-1–Flag and (Strep)2–AP2XI-2 in insect cells, with AP2IX-6–Flag serving as an internal control (Fig. 5c). AP2XII-1 was purified by Strep-Tactin affinity chromatography, and the partnership between AP2XII-1 and AP2XI-2 was confirmed through western blot analysis (Fig. 5d), whereas no co-enrichment was detected with AP2IX-6 (Fig. 5e). Consistent with AP2XI-2 and AP2XII-1 being part of a heterodimer, our findings show these two proteins coelute in the same gel filtration fractions, in a MORC- and HDAC3-independent manner, as confirmed by MS-based proteomics (Fig. 5f,g and Supplementary Table 5). Many transcription factors, including apicomplexan AP2, were reported to form homodimers and heterodimers with different partners that modulate DNA-binding specificity and affinity34,35. In this context, AP2XI-2 and AP2XII-1 probably bind cooperatively as a heterodimer to DNA to selectively and synergistically repress merozoite gene expression, and only their simultaneous depletion leads to achievement of the developmental program critical for merozoite formation.
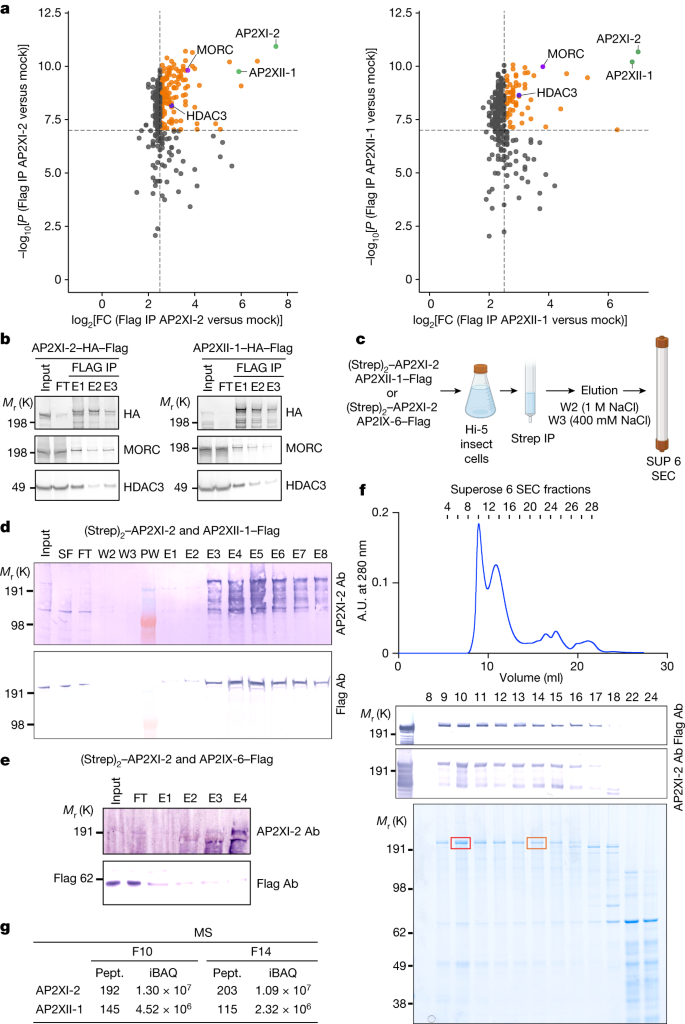
a, Flag immunoprecipitation (IP) eluates in HFF cells infected with wild-type parasite (mock) or parasites stably expressing HA–Flag-tagged AP2XII-1 or AP2XI-2 protein were examined through label-free quantitative proteomics using MS (three replicates per condition) and are represented as a volcano plot. Each dot represents a protein. Black dashed lines show −log10[P value] < 7 and log2[FC] = 2.5 cutoffs; proteins above these thresholds are coloured in orange, with specific proteins in blue, purple and green as indicated. Raw data and a detailed statistical analysis are shown in Supplementary Table 4. ...
Comments
Post a Comment