Interaction between tissue-dwelling helminth and the gut microbiota ... - Nature.com
Abstract
Tissue-dwelling helminths affect billions of people around the world. They are potent manipulators of the host immune system, prominently by promoting regulatory T cells (Tregs) and are generally associated with a modified host gut microbiome. However, the role of the gut microbiota in the immunomodulatory processes for these non-intestinal parasites is still unclear. In the present study, we used an extra-intestinal cestode helminth model-larval Echinococcus multilocularis to explore the tripartite partnership (host-helminth-bacteria) in the context of regulating colonic Tregs in Balb/c mice. We showed that larval E. multilocularis infection in the peritoneal cavity attenuated colitis in Balb/c mice and induced a significant expansion of colonic Foxp3+ Treg populations. Fecal microbiota depletion and transplantation experiments showed that the gut microbiota contributed to increasing Tregs after the helminth infection. Shotgun metagenomic and metabolic analyses revealed that the gut microbiome structure after infection was significantly shifted with a remarkable increase of Lactobacillus reuteri and that the microbial metabolic capability was reprogrammed to produce more Treg cell regulator-short-chain fatty acids in feces. Furthermore, we also prove that the L. reuteri strain elevated in infected mice was sufficient to promote the colonic Treg frequency and its growth was potentially associated with T cell-dependent immunity in larval E. multilocularis infection. Collectively, these findings indicate that the extraintestinal helminth drives expansions of host colonic Tregs through the gut microbes. This study suggests that the gut microbiome serves as a critical component of anti-inflammation effects even for a therapy based on an extraintestinal helminth.
Introduction
Helminths are multicellular parasitic worms that affect one-third of the global population worldwide. They can lead to chronic infections by modulating host type 2 immune responses, thereby avoiding immune-mediated expulsion1. Chronic helminth infection has been associated with reduced prevalence of allergy, autoimmunity, and inflammatory bowel disease in human populations or suppression of these diseases in animal models2. Therefore, understanding how helminths manipulate host immune immunity is of utmost importance for exploring ways to treat allergic or inflammatory conditions, a growing burden on the healthcare system in developed and even developing countries.
Typically, the modulation of helminth infection on host Th2 immune responses involves the suppression of multiple immune cell types through the release of various of excretory-secretory (ES) products3. One of the most prominent pathways for helminth immunomodulation is promoting regulatory T cell (Treg) expansion4. Tregs have central roles in preventing immune dysregulation and protecting the host from pathogenic immune responses5. Studies have suggested that helminths induce Tregs either directly by secreting factors or indirectly by interacting with bystander cell types such as dendritic cells and macrophages that induce Tregs. In addition, increasing evidence suggests that cross-talk with the local microbiota also has an important role4,6. Recently, the causal relationship in which intestinal microbiota contributes to the ability of intestinal helminths to modulate allergic disease or inflammation through promoting Treg expansions has been established7,8. Several studies have shown that infection with Heligmosomoides polygyrus induces significant alterations in the microbiota3 and correlates with the increase in short-chain fatty acid (SCFA) production8, which promotes immune regulatory response by stimulating the differentiation and suppressive capacity of Foxp3+ Tregs in the intestine9.
The mechanisms underlying the induction of Tregs by the gut microbiota have almost exclusively been defined from studies on the intestine-dwelling or submucosa helminth models (i,e., H. polygyrus, Trichuris muris and Hymenolepis diminuta)8,10,11,12. Due to colonization of the intestinal lumen, the reported quantitative and qualitative changes in the gut microbiota composition following these intestinal helminth infections may be associated with direct interplay between the gut microbiota and helminth (or helminth ES products), in which the direct interventions from secretions or collateral mucosal tissue damages might potentially confound the research observations. Tissue-dwelling helminths or helminths with an extra-intestinal phase of their lifecycle, represent a large proportion of the whole helminth population, including many nematodes, cestodes, and trematodes, and can change the host gut microbiota. For example, Schistosoma mansoni13, larval Echinococcus granulosus14 and Trichinella spiralis15 have been reported to be associated with the alterations of the gut microbiota in the host. However, whether these gut alterations participate in mechanisms of Treg differentiation in these tissue-dwelling helminth infections is yet to be ascertained.
In this study, we used an extra-intestinal cestode helminth-larval (metacestode) stage of Echinococcus multilocularis to explore whether there is an interaction between tissue-resident parasites and the host gut microbiota to modulate host mucosal immunity through promoting Tregs. E. multilocularis is the causative pathogen for alveolar echinococcosis, and its infection in the peritoneal cavity of mice serves as a good experimental model in molecular host-parasite interplay16. Our results showed that larval E. multilocularis infection could modulate the host mucosal immunity through a cross-talk with the gut microbiota, in which the Lactobacillus reuteri isolate is expanded and able to promote Treg expansion. This study established the mutualistic relationship between host gut microbiota and the promotion of Treg cells in a tissue-dwelling helminth.
Results
Chronic Echinococcus multilocularis infection reduces colitis in Balb/c mice
Consistent with the findings of previous studies suggesting that helminth infections are associated with remission of intestinal inflammation1,17, we observed that E. multilocularis larvae (Emu) infection reduced intestinal mucosal damage and inflammatory responses in DSS-induced colitis in the Balb/c mouse model (Fig. 1). Inflammation for the Balb/c mice at 90 days post-Emu infection (Fig. 1a, b) was triggered by dextran sodium sulfate (DSS) administration in drinking water. As expected, DSS treatment resulted in a significantly higher Disease Activity Index (DAI) score (Fig. 1c), reduced weight (Fig. 1d), shortened colon length (Fig. 1e, f), and mucosal damage and mucosal erosion (Fig. 1g, h) in the naive group. In contrast, the mice infected with Emu suffered a less severe inflammation with a reduction in weight loss, disease activity, colon shortening and pathologic damages (Fig. 1c–h). These results indicate that the infection of this extra-intestinal worm-larval E. multilocularis is associated with colonic immunoregulation and reduction of colitis in this model.
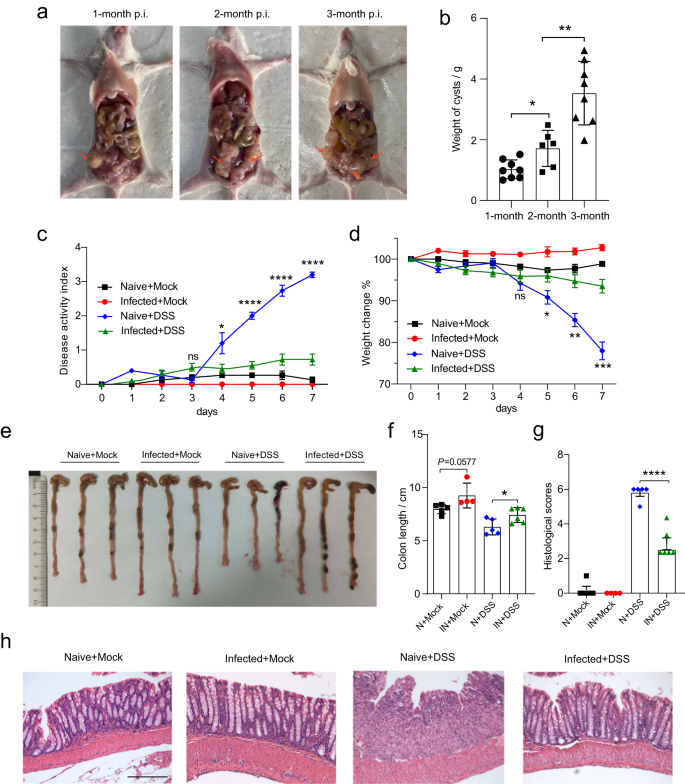
The experiments were conducted on mice on day 90 post-infection (p.i.) of Emu in the peritoneal cavity (panels (a, b)). The Balb/c mice were treated with either 5% DSS or Mock (autoclaved water) for 7 days. Larval E. multilocularis infection (on day 90 post-infection) in the peritoneal cavity attenuated the colitis in Balb/c mice (N Naïve, IN Infected), in the context of disease activity index (DAI) (c), weight loss across the experiment (d), colon length (e, f), inflammation scores estimated from distal colon tissue with H&E staining (g), and representative H&E staining sections of distal colon tissues (×100; The scale bar represents 200 µm in length) (h). Experiments were repeated 2 times with similar results. The data are shown from a representative experiment (mean ± SD shown). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using the two-sided Student t-test (n = 6–8 mice per stage for panel (b) and n = 4–6 per group for panels (c–h)).
Chronic Emu infection induces expansion of colonic regulatory T cells
Tregs have central roles in helminth-mediated gut immune regulation18,19. We found that CD4+ T cells from Emu-infected mice at day 90 post-infection were oriented towards a Treg pathway (CD4+CD25+) at either spleen or colon site (Fig. 2a). Forkhead box P3 positive (Foxp3+) Treg cells are the pivotal Treg populations that are critical for immune homeostasis and inflammation control. In keeping with the data in the DSS-induced model, Tregs at lamina propria (LP) of the colon site showed a significantly higher proportion of T cells with the expression of Foxp3+ (CD4+CD25+Foxp3+; Fig. 2b and Supplementary Fig. 1), indicating that the developments of Tregs following Emu infection were promoted at the colon site. Specifically, the frequencies of CD4+CD25highFoxp3+ T cells but not CD4+CD25lowFoxp3+ were significantly elevated in these mice (Fig. 2c). CD103 is a well-established marker for murine effector/memory-like Treg cells which is expressed following their activation in infectious diseases20. We observed a marked increase in the proportion of colonic CD103+Foxp3+ Treg cells within the CD4+ T cells in the Emu-infected mice (Fig. 2d). Collectively, these data suggest that Emu infection potentially raises the activated colonic Foxp3+ Treg cell populations.
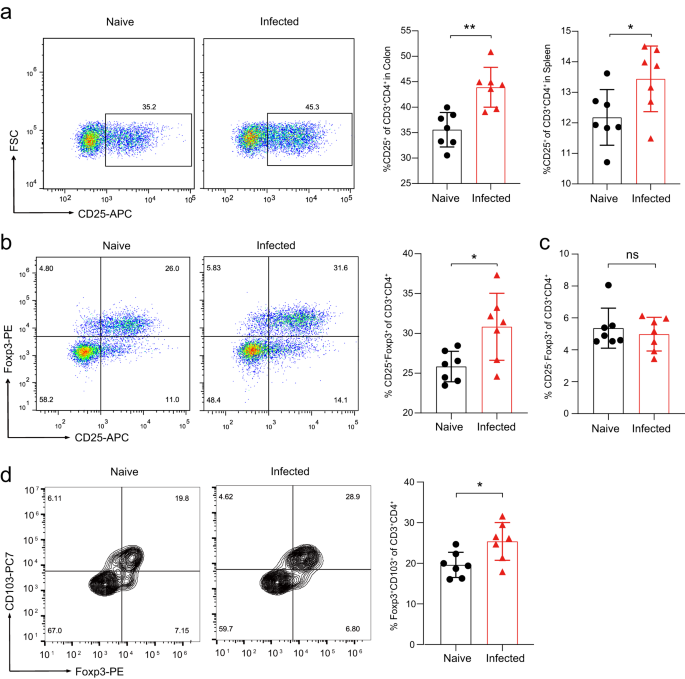
On day 90 post-infection of Emu, the mice were euthanized and subject to flow cytometry analysis of colonic (lamina propria) and splenic lymphocytes. a Expression of CD25 by immune cells isolated from the colon of naive and Emu-infected mice (left), and proportions of CD25+ T cells in CD3+CD4+ cells in colon or spleen (right). b Expression of CD25 and Foxp3 by immune cells isolated from the lamina propria of the colon in naive and Emu-infected mice (left), and the proportion of colonic CD25+Foxp3+ T cells in the CD3+CD4+ cell population (right). c The proportion of colonic CD25-Foxp3+ T cells in the CD3+CD4+ cell population. d Expression of CD103 and Foxp3 by immune cells isolated from the lamina propria of the colon in naive and Emu-infected mice (left), and the proportion of colonic CD103+Foxp3+ T cells in the CD3+CD4+ cell population in LP (right). Flow cytometry data are representative of n = 7 mice per group. Experiments were repeated 2 times with similar results. The data shown are from a representative experiment (mean ± SD shown). ns, not significant. *P < 0.05, **P < 0.01, using the two-sided Student t-test.
The gut microbiota promotes Treg cells differentiation in Emu infection
As the gut microbiota is a potent inducer of intestinal Foxp3+ Treg cells in the colonic mucosa21, we next tested whether the gut microbiota contributes to the expansion of colonic Foxp3+ Treg during Emu infection. First, we depleted the gut microbiota of Naive or Emu-infected mice with an antibiotic cocktail (ABX) regime and analyzed the colonic Treg populations in these mice. The antibiotic treatment significantly depleted the gut microbes in these mice (Supplementary Fig. 2). The colonic Foxp3+ Treg (or CD4+CD25+) ratio within CD3+CD4+ T cell population in the infected mice with ABX treatment was significantly reduced in comparison with the infected mice treated with vehicle control, albeit higher than that in naive mice with ABX treatment (Fig. 3a). These data suggest that the proliferation of Foxp3+ Treg cells induced by infection with Emu is tightly linked with the gut microbiota.
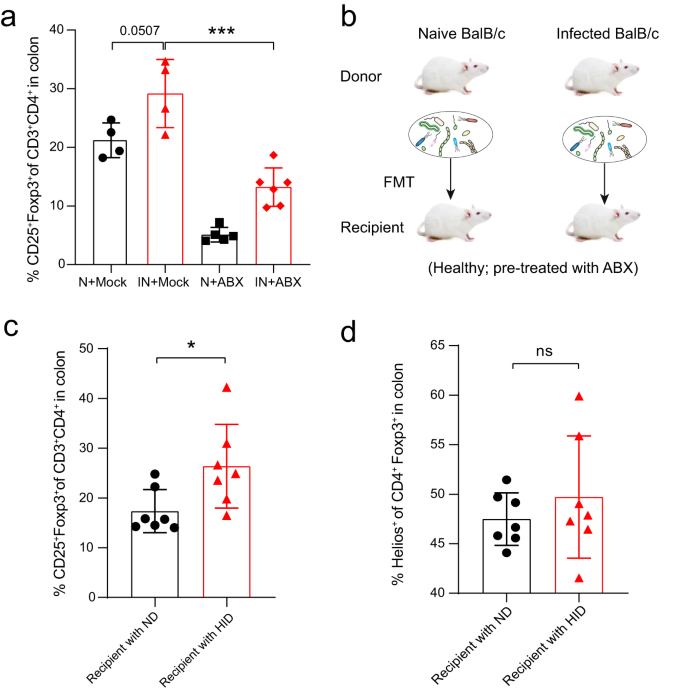
a After depletion of the gut microbiota with antibiotic cocktail treatment (ABX), the proportion of colonic CD25+Foxp3+ Treg cells in the CD3+CD4+ cell population in Emu-infected (IN) mice was significantly decreased in comparison with that in the infected mice treated with Mock. Naive mice (N) with or without ABX treatment were included as controls. b Schematic showing the experimental design for fecal transplantation transfer (FMT). Recipient mice were pretreated with ABX cocktails for 2 weeks, followed by an FMT regime of 2 weeks from naive donors (ND) or helminth-infected donors (HID). c The proportion of colonic CD25+Foxp3+ Treg cells in the CD3+CD4+ cell population by flow cytometry analysis for the recipient mice. d The proportion of colonic Helios+ Treg cells in Foxp3+ Treg cells by flow cytometry analysis for the recipient mice. The flow cytometry analysis was performed at 4 weeks post-FMT engraftment. Experiments were repeated at least 2 times with similar results. The data shown are from a representative experiment (mean ± SD shown). ns, not significant; *P < 0.05, ***P < 0.001 using the two-sided Student t-test (n = 4–6 mice for panel a; n = 7 for panels (b–d)).
We therefore further investigated the role of gut microbes through fecal microbiota transplantation (FMT). We transferred the fecal microbiota of either naïve or Emu-infected mice at day 90 post-infection into groups of healthy mice that had been pretreated with an antibiotics cocktail for 2 weeks (Fig. 3b). As the tissue-resident parasite lives in the peritoneal cavity of the infected mice, no parasite was transferred. At 4 weeks after FMT engraftment, the colonic Foxp3+ Treg frequency in the recipient mice that were transplanted with the gut microbiota from helminth-infected donors (HID) was significantly enhanced in comparison with that in the mice treated with the gut microbiota collected from the naive donors (ND) (Fig. 3c). These data suggest that the gut microbiota contributes to the expansion of colonic Foxp3+ Tregs in the mice with Emu infection. Previous studies have shown that gut microbiota induces the expansions of Tregs already present in the colonic LP, regardless of its origin9. In accordance with this finding, we observed a comparable frequency of Tregs that co-express Helios (Helios+, which indicates a thymic origin) between recipient mice transferred with the gut microbiota from ND and HID (Fig. 3d). Interestingly, the gut microbiota from HID failed to induce a higher proportion of CD25+Foxp3+ Tregs (Supplementary Fig. 3) in the spleen when compared with that in the mice transplanted with microbiota from ND. Taken together, these results suggest that the gut microbiota promotes intestinal mucosal Treg expansions during larval E. multilocularis infection.
The gut microbiome is skewed with increased L. reuteri after Emu infection
The above results strongly suggest that the extraintestinal helminth infection induced alterations in the gut microbiota. Next, we used shotgun metagenomics to compare the species-level microbiome compositions of the fecal samples collected from the naive mice and Emu-infected mice at day 90 post-infection. Our data revealed that Emu infection induced significant compositional changes in the gut microbiota (Fig. 4a–d). As indicated by the Principal component analysis (PCA) (Fig. 4a, P < 0.01, PERANOVA test), the microbiota community structure in infected mice was significantly skewed in comparison with that of naive mice, whereas no significant changes in alpha diversity were observed (Fig. 4b). At the phylum level, the Emu infection induced an expansion of Firmicutes and a reduction of Bacteroidetes (Fig. 4c). At the species level, the differential analysis revealed that L. reuteri was the most differentially abundant bacterium to discriminate naive against infection (Fig. 4d). The relative abundances of L. reuteri were much higher in mice infected with Emu than in naive, with an average fold-change of more than 100 (Fig. 4e). These data suggest that Emu infection resulted in a significant alteration of the gut microbiota and a striking expansion of L. reuteri.
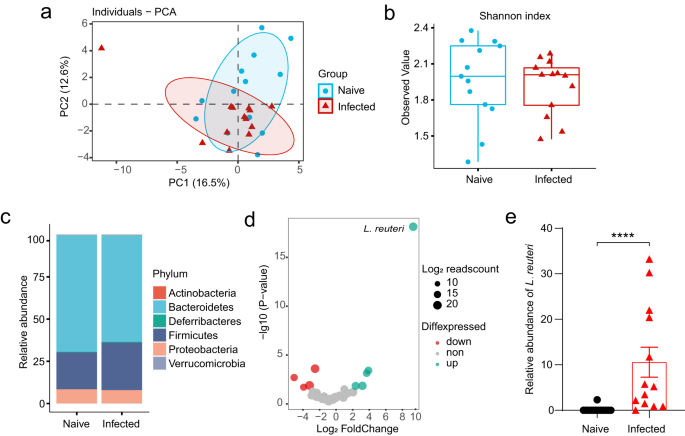
The shotgun metagenomic analysis of fecal samples was collected from the naive (n = 13) or Emu-infected mice (n = 13) on day 90 post-infection. a Principle component analysis (PCA) for the gut microbiome of the samples based on the relative abundance of each taxon at the species level (R2 = 0.14, P = 0.003). b Shannon index of alpha diversity (P > 0.05). c The relative abundances of taxa at the Phylum level. d Differential abundance analysis of the gut microbiota components at the species level. Log10-transformed P-values are shown on Y-axis. The colors of the points indicate whether a taxon in the Emu-infected group was over-represented (up), under-represented (down) and unchanged (non) in comparison with that in the naive group. e The relative abundance of L. reuteri (mean ± SD shown). The significance of PCA analysis for panel a was determined by Permutational multivariate analysis of variance (PERANOVA). ****P < 0.0001 using the two-sided Mann-Whitney-test for panels (b, e). Data in boxes of panel (b) represent the 25th percentile, median, and 75th percentile and whiskers stretch to 1.5 times the interquartile range from the corresponding hinge.
Metabolic reprogramming of the gut microbiota increases short-chain fatty acids
We wondered if the changes in microbial community structure would translate into altered microbial metabolism. PCA of metabolic pathway abundance data for the gut microbiome showed a significant separation (P < 0.05) between animals uninfected and infected with Emu (Fig. 5a). Differential abundance analysis identified 38 global pathways and 93 pathways at the species level that were significantly altered after infection (Supplementary Data 1) (FDR < 0.05), including several involved in carbohydrate metabolism and short-chain fatty acids (SCFAs) synthesis (e.g., 'pyruvate fermentation to butanoate' Fig. 5b). Of note, microbiota-derived short-chain fatty acids (SCFAs) are well-known anti-inflammatory mediators and Treg inducers for host intestinal immunity9. Using targeted metabolomics, we measured the levels of seven SCFAs in the stool samples of the mice. Consistent with observations in the in silico metagenomic analysis, we found that levels of fecal SCFAs (acetate, propionate, and butyrate) were significantly elevated in mice infected with Emu (Fig. 5c), indicating an enhanced capability of SCFAs formation of the gut microbiota in the Emu-infected mice.
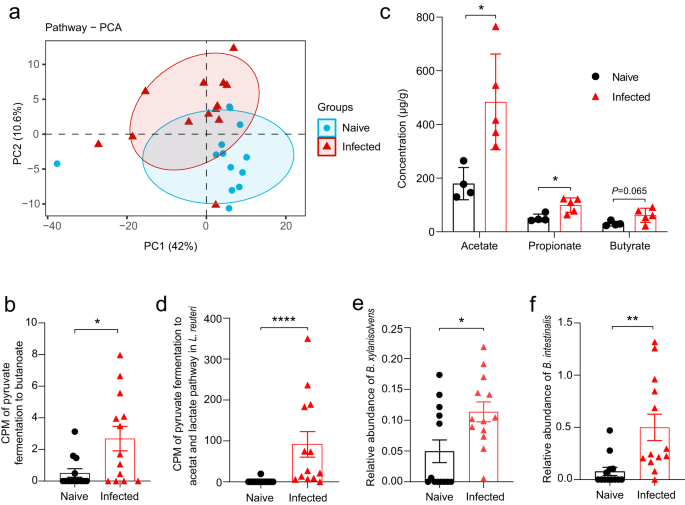
The abundance of each pathway in the shotgun metagenomic analysis was evaluated by HUMAnN2. a Principle component analysis (PCA) for the gut microbiome based on the relative abundance of each pathway at the species level (R2 = 0.22, P = 0.01). b Counts per million (CPM) of the pathway of 'pyruvate fermentation to butanoate' of the microbiome in the MetaPhlan analysis. c The concentrations of short-chain fatty acids of fecal samples in the metabolomic analysis (mean ± SD shown). d CPM of the pathway of 'pyruvate fermentation to acetate and lactate' in an L. reuteri isolate in the MetaPhlan analysis. e Relative abundances of Bacteroides xylanisolvens and (f) Bacteroides intestinalis. The significance of PCA analysis for panel a was determined by Permutational multivariate analysis of variance (PERANOVA). ns, not significant. *P < 0.05, **P < 0.01, ****P < 0.0001 using the two-sided Mann-Whitney-test for panels (b), and (d–f) (n = 13 for naïve mice and n = 13 for infected mice), and using the two-sided Student-t test for panel (c) (n = 4–5 per group).
Specifically, the metabolic profiling at the species level based on shotgun metagenomic data revealed that the pathway of 'pyruvate fermentation to acetate and lactate' in an L. reuteri isolate was significantly increased in abundance (Fig. 5d). Although L. reuteri might generally not be capable of direct formation for propionate and butyrate, it facilitates the production of these SCFAs by providing acetate and lactate as substrates for colonic SCFA producers, e.g., Bacteroides spp22. Consistent with this notion, we observed an increase of Bacteroides species (i.e., Bacteroides intestinalis and Bacteroides xylanisolvens) after Emu infection (Fig. 5e, f). We isolated the elevated L. reuteri strain (confirmed by 16 S rRNA sequencing) from Emu-infected mice and then gavaged the L. reuteri isolate to healthy Balb/c mice. SCFAs were significantly increased in the feces after the administration (Supplementary Fig. 4). Collectively, the results suggest that an L. reuteri-related metabolic shift and increased SCFA levels are hallmarks of the gut microbiome after Emu infection.
L. reuteri isolate enhances Treg frequencies and is promoted by Emu infection
Together with evidence from microbiota depletion and FMT experiments (Figs. 3 and 4), these data suggest that the microbial alterations marked by L. reuteri expansions contribute to the elevation of Tregs. In accordance with the notion, we found that the Tregs ratio was not altered 6 weeks after infection (Supplementary Fig. 5), where the gut microbial structures (Supplementary Fig. 6a) and the relative abundance of L. reuteri were slightly changed (Supplementary Fig. 6b), which indicates that alterations in microbiota and L. reuteri occurred earlier than the Treg expansions. Although certain L. reuteri strains have been shown to have the capability to promote Tregs23,24, a high variation for this capability usually exists among bacteria isolates. To test whether the isolated L. reuteri strain could promote Tregs, this bacterium and other controls (Escherichia coli, B. intestinalis, and B. xylanisolvens) were administrated to uninfected mice by gavage for 2 weeks (Fig. 6a). qPCR analysis of fecal samples showed successful colonization of these bacteria isolates in the gut of these mice after administration (Fig. 6b). The frequency of Foxp3+ Tregs was significantly elevated in the mice treated with the L. reuteri isolate. However, this Treg expansion was not observed in the mice administrated with any other bacteria (i.e., Escherichia coli, B. intestinalis, and B. xylanisolvens) (Fig. 6c). Hence, this data suggests that exposure of the L. reuteri isolate alone is sufficient to promote Treg frequencies in the host.
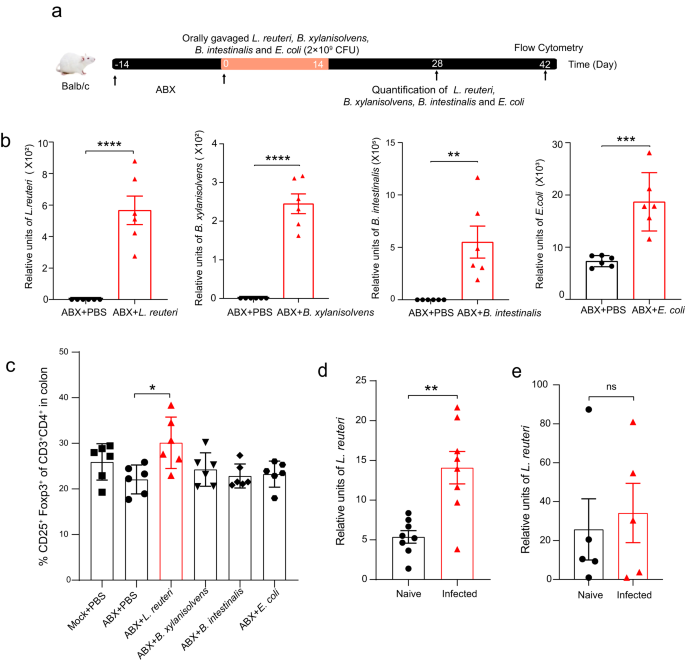
a The schematic showing the experimental design for L. reuteri and other control microbe administration experiments. The mice were orally gavaged with each isolate daily for 2 weeks (on days 0–14) after antibiotics (ABX) treatment. b The level of each bacterium was measured by qPCR on day 28. c The frequency of colonic Foxp3+ Treg cells was quantitatively measured by Flow cytometry on day 42. The L. reuteri isolate (2 × 109 CFU per mouse) was administrated to the mice on day 60 post-Emu infection for panels d-e and the levels of the bacterium in feces were detected. d The level of L. reuteri in naive or Emu-infected Balb/c mice. e The level of L. reuteri in naive or Emu-infected Nude Balb/c mice. Data for each sample are presented as the mean expression level in arbitrary units. Experiments were repeated at least 2 times with similar results. The data shown are from a representative experiment (mean ± SD shown). ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using the two-sided Student t-test for panels (n = 6 mice per group for panels b, c; n = 8 for panel d; n = 5 for panel e).
The above data indicate that L. reuteri is associated with the host gut immunoregulation after an extra-intestinal helminth infection. Interestingly, we observed that the L. reuteri expansion might be promoted by host immunity in return. Naive and Emu-infected Balb/c mice received L. reuteri isolate by oral gavage (on day 60). We detected the level of L. reuteri in the mice post gavage and found that the L. reuteri isolate level was much higher in the mice that were Emu-infected (Fig. 6d). In contrast, we did not observe a significantly increased level of the L. reuteri isolate in the Emu-infected Balb/c Nude mice (which are T cell-deficient and were treated the same way) when compared with their naive counterpart (Fig. 6e). These preliminary results raise a possibility that T cells might be linked with the L. reuteri overgrowth in the gut of Emu-infected mice.
Discussion
Tregs play a central role in the immunomodulatory effect of helminths2. The mechanisms underlying the inductions of Tregs by helminth are only partially understood. Increasing evidence shows the essential contribution of commensal microbiota to the induction and accumulation of the colonic Treg cell population25. Currently, the causal link between the gut microbial commensals and the regulatory T-cells in helminth infection has been mainly established in the intestinal helminth model species H. polygyrus7,8,26. Tissue-dwelling helminths or helminths with an extra-intestinal phase of their life cycles, such as filarial parasites, flukes and tapeworms, represent a large proportion of the helminth population and affect billions of people worldwide27. Therefore, it is of utmost importance and interest to dissect the roles of gut microbes in the immunoregulatory effects conferred by these parasites. In this study, we reported that the gut microbiota influences Treg populations, even in an extra-intestinal helminth. Therefore, our results suggest that the involvement of gut microbiota is likely a universal mechanism in helminth-induced regulatory immune responses.
One of the most striking findings in this study is the establishment of the causal link between the expansion of colonic Foxp3+ Tregs and the gut microbiota for a tissue-dwelling helminth. In spite of substantially different modes of interaction with the gut mucosa (e.g., luminal vs. tissue-dwellers) for these helminths3,28, one aspect that is often overlooked is that helminth-associated changes in gut microbiota composition occur29. It is very likely that the three-way interactions universally occur between helminth parasites, the resident microbes, and the host gut. In this study, we confirmed this notion in the context of mucosal immunity in a larval cestode infection in Balb/c mice. Consequently, more consideration needs to be taken on the effect of the gut microbiota when evaluating the influence and therapeutic potential of an extra-intestinal helminth. In addition, the findings observed from this tissue-dwelling helminth support that the shift in commensal microbiota is more likely (at least partially) the response to helminth infection rather than directly driven by helminth secretions or collateral tissue damage. Our results (Fig. 2a) also suggest that multiple cooperating mechanisms between helminth and the gut microbiota might drive the mucosal immunoregulation during helminth infection, as the Tregs were still expanded even after antibiotics treatment during Emu infection. This is anticipated and consistent with the findings in extensive previous studies that helminth excretory-secretory (ES) products or induced-cytokines (e.g., TGF-β) are able to directly induce naive CD4+ T-cells to become Tregs4.
In this study, we observed that the microbial shift is marked by an expansion of an L. reuteri isolate. Our results indicate that the L. reuteri isolate is able to promote SCFA levels and Treg expansions. Although SCFAs are well-known strong inducers of Tregs, these findings are not sufficient to establish a causal link between L. reuteri and Treg expansion during Emu infection. Further research is needed to fully clarify this issue. In fact, the expansion of lactobacilli populations upon helminth colonization is one of the most frequently reported observations in different interaction systems between host and parasite species, such as H. polygyrus in mouse7,17, and T. spiralis in mice15, Ascaris suum in pig30, and Toxocara cati in cat31. Of note, certain Lactobacillus species or strains have been reported to exert beneficial therapeutic effects on many autoimmune-related diseases (e.g., allergic airway response, asthma, and allergy contact dermatitis) by augmenting host regulatory T cells23,32,33. In addition, the expansion of a Lactobacillus species (L. taiwanensis) was proved to promote the establishment of H. polygyrus infection via the enhancement of Treg-mediated responses in C57BL/6 mice7. These data suggest that the cross-talk with lactobacilli commensals is potentially a universal strategy employed by helminths to promote their persistence for long-term survival under host immune system attack, which in turn brings beneficial effects to their hosts under inflammatory or pathogenic conditions. In agreement with this view, an alteration of microbiota-derived immunoregulatory metabolites in helminth infection is also frequently observed in many hosts parasitized by various helminths3. Among them, SCFAs are prominent in influencing multiple facets of immunity, including stimulating the differentiation and suppressive capacity of Foxp3+ Tregs in the intestine9. Helminth infection has been repeatedly reported to affect host carbohydrate metabolism for SCFAs, such as infection of H. polygyrus in mice8, Haemouchus contortus in goats34, and T. suis in pigs35. The findings in this study provide further support that SCFAs potentially act as common microbiota-derived signaling involving extraintestinal helminth-induced regulatory T-cell expansions. In this regard, these metabolites of gut microbiota appear to serve as messengers to facilitate cross-talk between the host and gut microbiota during helminth infections, which may be employed as a strategy to invade host immune systems and hence are profoundly implicated in host health and disease. However, the mechanism through which the helminth infection drives the changes in the microbiome, lactobacilli populations, and SCFAs is still not fully understood. Our results in the nude mice highlight the possibility that this expansion is likely T-cell-dependent. In accordance with this notion, the gut microbiome alterations during H. polygyrus and T. muris infections were observed to be Th2-associated10,36. Thus, the helminth infections might provide an immunity-dependent intestinal niche within the ecosystem of gut microorganisms in favor of the growth of these bacteria. In Fig. 6, L. reuteri seems to be much easier to colonize in the gut of Balb/c-Nude mice than in the normal Balb/c mice, which suggests that the absence of T cell-based immunity favors L. reuteri expansion. It is noteworthy that both nude mice and helminth-infected mice have impaired T-cell-based immunity and both have higher levels of L. reuteri. Thus, this observation together with the findings on Emu-infected mice is consistently in agreement with the notion that suppression or loss of T cell-based immunity might be linked with L. reuteri expansion. However, the initial gut microbiota structure might have effects on these preliminary observations and the exact mechanisms behind this observation remain elusive.
Overall, the results from this study demonstrated that larval E. multilocularis infection induces significant alterations in the host gut microbiome, which is marked by an increase in L. reuteri and SCFAs, and that these Emu-induced microbial changes contribute to the expansion of colonic Tregs induced by a tissue-dwelling helminth. These findings provide perspectives into the mutualistic relationships between a commensal microbe and a tissue-dwelling helminth parasite in terms of gut immunoregulation. A better understanding of the role of the gut microbiota in helminth-induced immune modulation would facilitate the development of novel therapeutics for inflammatory and autoimmune diseases that have important public health implications.
Methods
Mice and infections
Specific-pathogen-free (SPF) Balb/c mice (female, 7–9 weeks old) were purchased from the Laboratory Animals Center of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. SPF Balb/c-Nude mice (Balb/c, C000103, female, 7–9 weeks old) were obtained from Changzhou Cavens Lab Animal Co., Ltd (Jiangsu Province, China). All the animals were housed in an environment with a temperature of 22 ± 1 °C, and a light/dark cycle of 12/12 h. All animal studies (including the mice euthanasia procedure) were done in compliance with the regulations and guidelines of the Institutional Animal Care and Use Committee of Lanzhou Veterinary Research Institute (Reference NO. LVRIAEC-2019-020).
The E. multilocularis strain, isolated from Qinghai in China, was used in this study37. The E. multilocularis protoscolex (metacestode stage) was collected from hydatid cysts isolated from Mongolian gerbils (Meriones unguiculatus) that were intraperitoneally inoculated with larval E. multilocularis for five months according to the method previously reported38. Briefly, the metacestode tissue was cut into thin slices and strained through a tea strainer, followed by three washes with PBS supplemented with 1% penicillin/streptomycin. The larval E. multilocularis infection group for each experiment was intraperitoneally injected with E. multilocularis protoscolex (n = 2000) in 200 μL phosphate-buffered saline (PBS), and the mice in the control group were intraperitoneally injected with the same volume of PBS. The cysts generally live in the peritoneal cavity of the infected mice.
Dextran sodium sulfate-induced colitis and histology
To induce colitis, the Balb/c mice were given 5% (w/v) dextran sodium sulfate (DSS, MP Biomedicals) (MW = 36,000–50,000) in drinking water for 7 days. The DSS-containing drinking water was refreshed every three days and replaced with autoclaved water normally used in the animal facility on day 7. Mice in the control group were given autoclaved water only. Disease activity index (DAI) was calculated daily, and included loss of body weight data (0, 0–1%; 1, 1–5%; 2, 5–10%; 3, 10–15%; 4, 15–20%), stool performance (0, normal; 1, soft and shaped; 2, loose; 3, between; 4, diarrhea), and the presence of occult blood (0, no blood; 1, +; 2, ++; 3, +++; 4, ++++). The presence of occult blood was measured using the Fecal Occult Blood Test Kit (Leagene, China) according to the manufacturer's protocol. All the mice were euthanized on day 8. At sacrifice, colons were removed and measured in length. Approximately 1 cm of the distal colon was embedded into paraffin, after fixation in 10% buffered formalin at room temperature for 24 h. Colon sections were stained with hematoxylin and eosin (H&E) to examine histological alterations. Histological scores were evaluated using the criteria previously described39. Histology scoring was performed in a blinded fashion with a protocol as follows: cell infiltration: occasional inflammatory cells in the lamina propria (LP) (score 0); increased infiltrate in the LP predominantly at the base of crypts (score 1); confluence of inflammatory infiltrate extending into the mucosa (score 2); transmural extension of infiltrate (score 3). Tissue damage: no mucosal damage (score 0); partial (up to 50%) loss of crypts in large areas (score 1); partial to total 50–100% loss of crypts in large areas, epithelium intact (score 2); total loss of crypts in large areas and epithelium lost (score 3).
Antibiotic treatments and fecal microbiota transplantation (FMT)
A cocktail of antibiotics (ABX) (ampicillin, vancomycin, gentamicin, neomycin, and metronidazole) (Sigma-Aldrich, USA) was used to effectively eliminate intestinal bacteria40. The efficiency of antibiotics treatment was assessed by evaluating the total bacterial load in feces. Briefly, the genomic DNA of fecal samples was extracted using a QIAamp PowerFecal Pro DNA Kit (QIAGEN, Germany). DNA concentration was quantified using a Qubit 4 Fluorometer (Thermo Scientific) and a total load of bacteria was detected by 16 S denaturing gradient gel electrophoresis and determined by qPCR targeting the 16 S rRNA gene (primers F: 5′ -CGGYCCAGACTCCTACGGG-3′; R: 5′-TTACCGCGGCTGCTGGCAC-3)41, using the method in a previous study42. The qPCR procedure was performed using the SYBR Green-based method of GoTaq® qPCR Master Mix Kit (Promega, USA) according to the manufacturer's recommendation (Tm = 60 °C). Reactions were set up in triplicate and run alongside a serially-diluted pool of all samples to be analyzed to create a standard curve of Ct value vs. 16 S rRNA gene level in arbitrary units. Positive and negative controls were run on each plate. Data for each sample are presented as the mean level in arbitrary units and normalized by sample weight. To test whether the feces from ABX-treated mice lost the ability to produce SCFAs, which was reflected by inability to change the color of pH-sensitive Bromocresol purple agar (BCP) plates, feces collected from ABX-treated mice were homogenized, diluted, and cultured on BCP in an anaerobic chamber at 37 °C for 24 h. BCP is used to indicate acid production from whatever fermentable substrate is present in the medium. Yellow haloes are produced around acid-producing colonies.
For the gut microbiota depletion experiment in the mice pre-infected with Emu, the mice were treated by a protocol based on the 3 R principle. The antibiotic regimen (ad libitum in drinking water with 2.5% sucrose; ampicillin 1 g/L, vancomycin 0.5 g/L, gentamicin 1 g/L, neomycin 1...
Comments
Post a Comment