A practical guide for the diagnosis of abdominal angiostrongyliasis ... - Parasites & Vectors
Diagnosis of AA is generally confirmed by the identification of A. costaricensis eggs, larvae or adult worms during histopathological analysis of the vermiform appendix and small and large bowel [19], although rare cases of testicular and liver disease have also been reported [20,21,22]. It is also possible to confirm the diagnosis in suspected cases by polymerase chain reaction (PCR) using DNA from formalin-fixed paraffin-embedded tissues (FFPE) [23]. Nucleic acid detection in serum has also been standardized for AA using primers targeting A. cantonensis sequences [24, 25]. Studies are underway to design additional assays using A. costaricensis sequences.
Histopathology: macroscopic and microscopic findings
Macroscopically, an appendix infected with A. costaricensis is similar in appearance to that seen in routine cases of acute appendicitis, with red or black fibrinopurulent deposits in the serosa and a thickened wall (Fig. 2a, b) [13]. To increase the chances of finding parasitic structures during the microscopic analysis, when preparing the FFPE it is necessary to include the entire vermiform appendix and the mesoappendix [23].
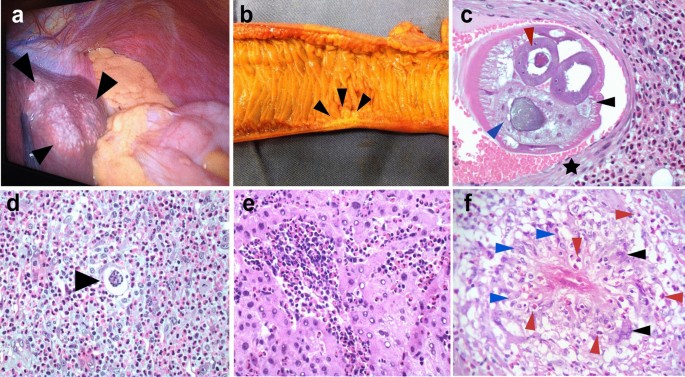
Macroscopic and microscopic histopathological findings of AA. a Extraintestinal angiostrongyliasis in liver with multiple small nodules (black arrowheads) and yellowish material. b Small bowel showing a segmental Crohn disease-like lesion with wall thickening and hemorrhagic area (black arrowheads). c Transversal section of Angiostrongylus costaricensis adult female worm inside a branch of the mesenteric artery showing polymyarian musculature (black arrowhead), uterus with an egg inside (red arrowhead) and gut (blue arrowhead). Muscle cells of the artery are shown by a black star [hematoxylin–eosin staining (HE), ×200]. d Angiostrongylus costaricensis egg (black arrowhead) inside a small vessel surrounded by severe eosinophilic infiltration (HE, ×400). e Eosinophilic infiltration in the liver of a patient with AA (HE, ×400). f Granuloma with histiocytes (blue arrowheads), giant multinucleated cells (black arrowhead) and eosinophils (red arrowheads) (HE, ×400)
Two types of macroscopic lesions are observed in the bowel: intestinal infarction, and segmental and nodular lesions in the large bowel with thickened areas. There may be multiple segmental and nodular lesions, which may resemble Crohn disease [26] (Fig. 2b). It is important to perform adequate sampling of the infarcted lesions by randomly selecting areas of the intestinal wall and undertaking extensive sectioning of the mesentery. If the diagnosis is not confirmed from the first sampling, it is necessary to embed the entire surgical specimen in paraffin since parasitic structures may be absent from some sections. Moreover, blocks of the entire macroscopic lesion are required when sampling segmental or nodular lesions. In these cases, samples of the mesentery should be taken only from the hemorrhagic sites or thickened vessels [23]. It is crucial to carefully analyze the antimesenteric portion of the bowel, where eggs or larvae may be found in capillary lumens [13].
Routine hematoxylin and eosin staining is used for microscopic analysis of AA. The diagnosis of AA is confirmed when eggs, larvae or sections of adult worms are found in the lumens of capillaries, arterioles and large arteries [5, 23]. Adult worms are found mainly in submucosal, muscular, serosa or large mesenteric/mesoappendix arteries, where they may be associated with thrombosis and infarction (Fig. 2c) [27, 28]. In our experience, the majority of adult worms are found in the submucosal and mesenteric arteries.
A type 2 inflammatory response induced by A. costaricensis generates a strong eosinophilic infiltration [5], a key observation during the microscopic examination of biopsies (Fig. 2c–f). Eosinophilic infiltration around capillaries and arterioles of the submucosa and muscularis propria is associated with severe disease. Eosinophilic infiltration of arterial walls, which is termed eosinophilic arteritis, is a histopathological feature of AA (Fig. 2c) [26]. Additionally, granulomas are observed in the walls of large arteries [26] or engulfing capillaries and arterioles, together with the presence of eggs or larva in the lumen [5, 29]. Severe granulomatous reactions in the submucosa and muscularis propria of the large bowel leads to pseudotumor formation together with small vessel occlusion due to inflammation. Necrosis of the mucosa or intestinal wall and secondary ulceration may occur in severe disease [29].
AA is associated with characteristic clinical manifestations in patients, the observation of eosinophilic infiltration, eosinophilic arteritis and granulomas engulfing capillaries and arterioles, as well as key epidemiological features. Sometimes it is necessary to include the complete surgical specimen in FFPE blocks and analyze serial slides of the suspected infected areas to identify parasitic structures.
In-house serological methods
In-house serological assays like a latex agglutination test (Morera test) and an immunoglobulin G (IgG)–enzyme-linked immunosorbent assay are available in Costa Rica (from the Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud, Cartago) and Brazil (from the Instituto Adolfo Lutz, São Paulo), and both use whole somatic A. costaricensis antigens [30]. Crude antigens from eggs have been evaluated for serological assays [31] but are not used in routine diagnostic tests.
Difficulties in obtaining large numbers of A. costaricensis adult specimens and maintaining the parasite's life cycle have prompted the use of whole crude antigens [32] and recombinant proteins (galectin) [33] from its congeneric species A. cantonensis for serological testing [32], which have proven successful. Since these assays use heterologous proteins of A. cantonensis, epidemiological factors as well as the patient's clinical history and manifestations should be considered for the correct interpretation of the results and to discard infection with A. cantonensis. It is highly likely that future protocols will involve rapid tests that use homologous recombinant antigens [33, 34]. Besides improving the reproducibility of these assays, highly purified and well-characterized A. costaricensis antigens may prevent cross-reactivity with other nematode species, and specifically to the threadworm Strongyloides stercoralis [31, 35]. Increased specificity may also result from the detection of IgG1 antibodies, as suggested by Abrahams-Sandi et al. [31]. The reactivity of human IgG decreases with time in post-acute infections, but it may remain detectable for several months [36].
PCR methods
Three DNA-based methods have been designed for confirming the diagnosis of AA. The first method was a conventional end-point PCR which used a 232-base pair fragment of a 66-kDa muscle protein of female A. cantonensis (Ac-fmp-1) as a target. This reaction detected the Ac-fmp-1 homologue in A. costaricensis [24] in the sera of two out of three patients with AA. Moreover, the PCR did not cross-amplify DNA of other gastrointestinal nematodes such as Strongyloides ratti, Ancylostoma caninum, Ascaris suum, and Toxocara canis [24].
The second conventional end-point PCR method used FFPE samples from patients with confirmed AA to detect the same Ac-fmp-1 homologous DNA fragment of A. costaricensis [23]. This method detected 55% (11/20) of cases confirmed by histopathology, especially in sections containing parasitic structures or granulomas [23]. Overall, this PCR showed intermediate sensitivity and high specificity, since the reactions were negative for FFPE samples of negative controls and FFPE samples with Ascaris lumbricoides, Enterobius vermicularis, Strongyloides stercoralis and Schistosoma mansoni [23].
The third molecular assay was a real-time PCR that also amplified a DNA fragment of the Ac-fmp-1 of A. costaricensis in sera of patients with presumptive AA [25]. This real-time PCR detected the parasite's DNA in two out of 28 sera matched to patients with AA. In addition, the two samples positive in the real-time PCR were negative according to an indirect enzyme-linked immunosorbent assay. Therefore, the method confirmed the presence of the nematode's DNA in sera of patients with suspected AA and complemented the results of serological techniques, suggesting that the assay might be useful during the acute phase of the infection [25].
Comments
Post a Comment