Investigating the etiologies behind emergent mass mortalities of farmed Liza carinata juveniles from coastal farms at Damietta, Egypt | Scientific Reports - Nature.com
Abstract
This study aimed to identify the mortality present in private fish farm Amyloodinium ocellatum and Cryptocaryon irritans were isolated from this outbreak affecting Liza carinata fingerlings at an earthen-based aquaculture facility in Damietta, Egypt. A total of 140 moribunds, L. carinata, were collected from the fish ponds during the mortality events. Physico-chemical analysis of water was analyzed. The skin, fins, gills, and eyes of each fish specimen were scraped gently onto slides in areas over 2 cm area. All smears were examined separately under the light microscope. Molecular identification of the parasites using analysis of ITS rDNA regions flanking both 18S and 28S rDNA genes of Amyloodinium protozoa and C. irritans. Identities of the detected parasites were confirmed by gene sequence and phylogenetic analysis. The majority of the examined fish (90%) were infected, 66.42% had a mixed infection, and 23.57% had a single infection either with A. ocellatum (10.71%) or C. irritans (12.85%).The mean intensity of A. ocellatum was 16.5 ± 2.03 in the skin and 13.18 ± 1.90 in the gills of infected fish, while that of C. irritans was 4.75 ± 1.05 in gills and 7.43 ± 1.45 in the skin, respectively. To control the emergent mortalities, affected ponds were treated using copper sulfate pentahydrate, hydrogen peroxides solutions, and amprolium hydrochloride powder in feed. Fish across the treated ponds were gradually improved with low morbidity and mortalityrates during the treatment period. The clinical disease was almost diminished at the end of the second week of treatment. Coinciding with the clinical improvement of the treated juveniles, microscopical examination of skin/gill scraps exhibited a marked decline in the number of protozoan parasites at the end of the second week of treatment.
Introduction
Marine aquaculture is a major economic industry in many countries. Egyptian mariculture sector is still in its early stages, and it is not fully developed as the freshwater aquaculture industry1. The need to expand the mariculture industry increases in Egypt due to the scarcity and limitation of freshwater resources. The enormous aquatic marine resources available in the country are anticipated to aid the prospected expansion of this sector2. Mariculture is mostly practiced in northern Egypt, especially in Damietta, Port Said, Alexandria, and the Suez Canal region2. Mullet, European sea bass, giltheadsea bream, and meager are the main cultured species3,4.
The family Mugilidae comprises 17 genera and around 72 species and is widely distributed worldwide. Mullets can withstand a wide salinity gradient and thrive in various environments, including marine, brackish, and even freshwater5. Egypt is a leading country in mullet culture. In the last 10 years, the Egyptian mullet aquaculture sector has expanded from approximately 130,000 T in 2012 to 242,061 MT in 20183. Egyptian hieroglyphics depict locals fishing for mullets over a thousand years ago (2340 B.C.)6. The availability of wild fry sources and extensive water resources (both brackish and marine) has encouraged the rapid growth of the mullet aquaculture industry in Egypt. Six different grey mullet species are commonly cultured in Egypt; flathead grey mullet, Mugil cephalus, thick lip grey mullet, Chelon labrosus, golden grey mullet, Liza aurata, black keeled mullet, Liza carinata (L. carinata), thin lip mullet L. ramada, and leaping mullet, Liza saliens. Farming of Mullet in Egypt still relies on collecting wild seed since induced spawning is only done on a small scale6. L. carinata (Valenciennes, 1836), sehlia, is known to inhabit the east coast of the Mediterranean, arriving through theSuez Canal from the Red Sea, its original distribution7. L. carinata is smaller and has a slower growth rate than other mullet species, yet there is a high market demand in Egypt, leading to high prices8,9.
Protozoan parasites are renowned threats to mariculture operations, causing massive financial losses that necessitate effective control measures. They have the potential to devastate fisheries and have a significant impact on fish production. Amyloodinium ocellatum (A. ocellatum) and Cryptocaryon irritans (C. irritans) are two serious protozoan parasites that cause severe mortality in wild and cultured fish10. A. ocellatum is an obligate ectoparasitic marine dinoflagellate that parasitizes a broad range of marine and brackish water fishes, causinghigh mortalities.The parasite causes the skin of affected fish to become powdery or velvety, and the resultant illness is known as velvet disease or amyloodiniosis11,12. It mostly infects the gills, skin, fins, eyes, and buccal cavity of host fish13.
The life cycle of A. ocellatum is divided into three stages, trophont, tomont, and a flagellate dinospore, infective stage14,15 A. ocellatum attaches itself to the epithelial tissues of the fish host through rhizoids, inflicting severe physical damage to the cells, culminating in hyperplasia, inflammation, bleeding, and necrosis11. Significant fish mortalities occur due to osmoregulatory imbalance and subsequent bacterial infections resulting from parasite feeding activity and the detachment of large numbers of trophonts16. Parasite feeding activities and the detachment of large numbers of trophonts cause severe osmo-regulatory imbalance and secondary bacterial infections collectively, resulting in substantial fish death11,16.
Cryptocaryon irritans parasites are obligate ectoparasitic protozoan that infects almost all marine teleosts17. C. irritans infects a broad range of wild and farmed fish species, causing cryptocaryoniasis or marine white spot disease with substantial losses, particularly in hatchery and nursery stages18. C. irritans multiplies rapidly and invades the integument of its host, significantly impeding skin and gill functioning19,20. The proliferation of epidermal cells induced by parasite feeding activities is evident macroscopically as white spots. Clinical signs of cryptocaryonosis in marine fish include pinhead-sized white nodules on the skin, fins, and gills. Fish also suffer from respiratory discomfort, pale gills, and excessive mucus production11. Cryptocaryon irritans parasites have a four-stage life cycle: theront, trophont, protomont, and a final tomont phase21. C. irritans produce lymphocytic infiltration, necrosis, and varying degrees of epithelial proliferation in fish's gills and skin, similar to Ichthyophthirius multifiliis21.
The control of parasitic fish diseases including, A. ocellatum and C. irritans in aquaculture, is complicated by the current limited availability of efficacious licensed products and the development of antiparasitic drug resistance. Therefore, the need to develop novel, safe, effective antiparasitic drugs is increasing. Copper sulfate, acriflavine, and formalin are commonly used to treat different fish parasitic infections, but these chemicals are highly toxic to fish22. Application of antiparasitic treatments in fish farming facilities requires awareness of aquaculture sustainability and environmental protection.
The present study aimed to investigate infections with protozoan parasites (A. ocellatum and C. irritans) in earthen ponds reared keeled mullet, L. carinata, during the early summer seasonof July 2020, using both morphological and molecular techniques. Further, the study aimed to evaluate the application of a combination of copper sulfate pentahydrate, hydrogen peroxides, Glutaraldehyde/QACs combination, and amprolium to control the mixed infections of A. ocellatum and C. irritansin a clinical field trial.
Materials and methods
Case history and fish sampling
In July 2020, L. carinata, keeled mullet, fingerlings reared in earthen ponds within a private farm at Shata, Damietta, Egypt, suffered from respiratory distress, and high mortalities were investigated. L. carinata was stocked with a density of 10,000 fish/acre.The mean weight of L. carinata at the onset of mortalities was 10 ± 2 g. the daily water replenishment rate was 20% of the total volume of the pond. The farm water has a reddish-brown colour. The feeding rate was 3% of the total fish biomass delivered three times during the day. The rice bran was the main feed delivered to fingerlings of L. carinata. The farm uses poultry and livestock manure. No Paddlewheels aerators exist on the farm. Fish fingerlings showed signs of respiratory distress, flashing, surfacing, accumulation at water inlet, and sudden death. L. carinata fingerlings were seen close to the margins of the ponds with a lack of escape reflex. Several dead fish were scattered through the pond's water and banks.
A total of 140 moribunds, L. carinata, with mean weight, 10 ± 2 g, were collected from the fishponds during the mortality events. Wet mounts were prepared and inspected on the spot at the farm. Scrapings was obtained from the gills and skin of moribund fish specimens. The fish were transferred to the Aquatic Animal Medicine and Management Laboratory, Faculty of Veterinary Medicine, Cairo University Egypt, in isothermal boxes with ice for further analysis.
Physico-chemical analysis of water
Dissolved oxygen (DO), temperature, pH, and turbidity, were measured in-situ in the selected fish ponds using a Multi-probe HQ40D meter (HACH LDO; PHC301 & CDC41, Germany). Salinity was recorded using a portable refractometer (ATAGO CO., LTD. Japan).Water samples were also collected and further analyzed for un-ionized ammonia (NH3), nitrite, and nitrate. Phytoplankton was collected, filtered through a 25 μm mesh, concentrated in 20 ml sterile seawater, and fixed with Lugol. Cells were counted using a Sedgwick Rafter S50 cell counter, and micro-phytoplankton counts were expressed as cells/ml according to methods described by23.
Parasitological examination
The skin, fins, gills, and eyes of each fish specimen were scraped gently onto slides in areas over 2 cm area. All smears were examined separately under the light microscope, X4 to X100, using an Olympus CX41 microscope, Japan, following the clinical procedures used by Noga11. Morphometric analysis of the parasitic protozoan depends on fifty parasites. All measurements are in micrometers in diameters and are given as mean S.D.24. Prevalence and mean intensity of protozoan infestations were calculated and recorded.
Molecular identification
The collected protozoan parasites; from the gills and skin of moribund fish; were washed several times with distilled water to remove tissue debris and mucus and then centrifuged at 2000 × g for 15 min. The pooled protozoan was transferred to sterilized Eppendorf tubes and preserved at − 20 °C for further molecular identification. DNA was extracted from preserved protozoan using QIAamp a DNA Mini kit (QIAGEN, Hilden, Germany). The concentration and quality of genomic DNA were investigated using the NanoDrop™ ND-1000 Spectrophotometer (Thermo Scientific, Germany). The parasitic DNA was then preserved at − 20 °C for sequencing analysis.
A fragment of ITS rDNA regions; flanking both 18S and 28S rDNA genes of Amyloodinium protozoan; was amplified using the following primer pair Dino 5′UF:5′-CAACCTGGTGATCCTGCCAGT-3′ and ITS R:5′-TCCCTGTTCATTCGCCATTAC-3′ as described by Levyet al.25. Briefly, PCR amplifications were performed using the following conditions: initial denaturation at 94 °C for 2 min, followed by 40 cycles of (94 °C for 30 s, 60 °C for 45 s, and 72 °C for 3 min), with a final extension at72 °C of 10 min. Amplicons were purified using the QIAGEN Extraction Kit protocol (Hilden, Germany). The purified amplicons were sent directly to the Macrogen sequencing company (Macrogen, Seol, South Korea) to be sequenced using ABI 3730XL DNA sequencer in both directions.
On the other hand, the amplification of the ITS- rDNA regions flanking 18S and 28S rDNA genes of the retrieved C. irritans was carried out using the following primers pair, P1-FW: 5′-GTTCCCCTTGAACGAGGAATTC-3′ and NC2-RV: 5′-TTAGTTTCTTTTCCTCCGCT-3′ as described by Niu et al.26. The amplification was started with an initial denaturation at 94 °C for 5 min, followed by 35 cycles of (94 °C for 30 s, 53 °C for 30 s and 72 °C for 1.5 min); with a final extension at 72 °C for 10 min. The amplicon was purified and sequenced as mentioned above using the same primer pair in both directions.
The Bio Edit program assembled and edited the two retrieved sequences27. The assembled sequences were aligned against other ITS rDNA regions of Amyloodinium and Cryptocaryon protozoan available in the database of GenBank. Finally, the sequenced ITS rDNA regions of Amyloodinium and Cryptocaryon protozoan were deposited in the GenBank.The neighbor-joining phylogenetic tree was constructed using MEGA X, with the following parameters: maximum likelihood parameter and 1000 bootstrap replicate28.
Field treatment trial
Farmed fish exhibited the same previously mentioned clinical signs in the earthen pond with a stocking density of around 8500/acre were treated with the following protocol.
The treatment strategy was divided into three main successive trials as follow.
- A.
Initial treatment: application of copper sulfate pentahydrate 99% at a dosage of 3 kg/acre were used as an initial disinfectant on daily basis for 7 successive days at 12 p.m.29, concurrently hydrogen peroxide 40% solutions were added at a dosage of 6.5 L/acre during the early mornings30.
- B.
Maintenance treatment: application of Glutaraldehyde (15%)/Quaternary ammonium compounds 25% (QACs) combination at a dosage of 200 ml/acre for 3 successive days in the late afternoon31. To decisively boost the treatment protocol, a systemic application of Amprolium HCl was used at a dosage of 190 g/ton feed for 3 successive days. The same treatment strategy was repeated after 2 weeks32.
- C.
Supportive treatment: at the end of the second week of treatment strategy, a supportive treatmentprotocol was adopted. Briefly, the addition of a mixture of vitamin C (1.5 kg/acre) and Saccharomyces cerevisiae (Brewer's yeast) (1.5 kg/acre) into the pond's water in the late afternoon as a weekly routine protocol to enhance pond aquatic biota as well as fish immune barriers.
A parasitological examination was conducted at 14 days of treatment. Fish mortalities and parasitic intensities in the skin and gills of treated fish were recorded. The intensity of protozoal infection based on mucous scrapping of skin/gills at the initiation of the treatment trial (0-day) and post two weeks treatment strategy were statistically compared by paired t-test using SPSS version26. A probability (P-value) of ≤ 0.05 was assumed for statistical significance.
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Cairo University, Egypt.
Accordance with relevant guidelines and regulations
Clinical examination, dissection, sampling, sample processing, microscopical examination, molecular typing methods and field treatment trials were carried out in accordance with relevant guidelines and regulations supported with relevant references throughout the manuscript materials and methods section.
Compliance with ARRIVE guidelines
The current study was carried out in compliance with the ARRIVE guidelines when relevant methods applied.
Results
Physicochemical analysis of water samples
The mean values of the physicochemical water parameters recorded in the earthen ponds were 30 °C, 32‰, 9, 3.4 mg/L, and 650.00 (NTU) for temperature, salinity, pH, dissolved oxygen, and turbidity, respectively. The average recorded levels of un-ionized ammonia (NH3), nitrite (NO2), and nitrate (NO3) were 1.3 mg/L and 0.98 mg/L 2.0 mg/L, respectively. The phytoplankton biomass averaged about 1675.0 (cells/mL).
Clinical examination
The fish showed typical symptoms of respiratory distress.Flashing, anorexia, sluggish movement, and fast opercular movement were all frequent. Fish aggregation at water surfaces and accumulation near water inlets were both common. Liza carinata fingerlings were seen close to the margins of the ponds with a lake of escape reflex. Excess mucus was seen in the gills of moribund fish. The skin of succumbed fish was hazy and velvety (Fig. 1), with white spots all over the body and around the eyes (Fig. 1). Some fish succumbed with no obvious gross lesions.
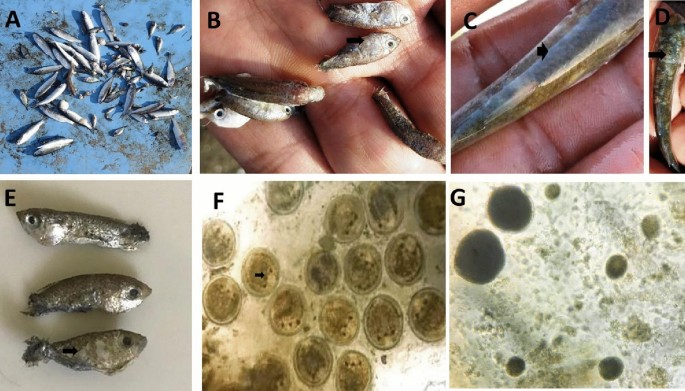
Mass mortality in Liza carinata fish with concurrent infection with Cryptocaryon irritans and Amyloodinium ocellatum infection in marine fish farm. (A) Mass mortalities in farmed Liza carinata; (B) white spots as pinhead-size on skin; fins and gills of small fish due to C. irritans; (C–E) sloughing of the skin of small fish with velvet formation due to Amyloodinium ocellatum; (F) C. irritans with the stage present on the scraping smears was the trophont, note the cytoplasm appeared opaque in color with 3–4 macronucleus, (G) Amyloodinium ocellatum small to large in size rounded to pear in shape with opaque and dark in color cytoplasm.
Prevalence of protozoan infections
The majority of examined fish (90%) were found infected. The greater part (66.42%) of fish examined had a mixed infection, while (23.57%) had a single infestation, either A. ocellatum (10.71%) or C. irritans (12.85%). The mean A. ocellatum intensity in fish tissues was (16.5 ± 2.03) in the skin and (13.18 ± 1.90) in the gills of infected fish. On the other hand, the mean intensity of C. irritans was (4.75. ± 1.05) in gills and (7.43 ± 1.45) in the skin of infected fish (Table 1).
Morphological identification
Amyloodinium ocellatum trophonts detected in the mucus scrapings were spherical to oval or pear in shape, ranging in length from 37 to 115 (69.76 ± 26 μm); the cytoplasm was opaque in colour, with rhizoids tentacle-like structure for firmly adhering to the gills (Fig. 1). The C. irritans trophont, on the other hand, was 325–475 μm (386 ± 1.5 μm) in length and 50–65 (62 ± 0.4 μm) in width. It was rounded to oval or pear in shape with opaque cytoplasm; small to large with crescent shape macronucleus containing four lobes with lengths of 6–10 μm (8.6 ± 0.42 μm) (Fig. 1).
Molecular identification
Amyloodinium ocellatum
The accession number of ITS rDNA regions of this Amyloodinium sp. infecting L. carinata was MZ710458. The length of the sequenced ITS region was 1318-bp. Depending on its sequence alignment, the present sequence is ascribed to species level to be identified as A. ocellatum and firmly embedded within the family Oodiniaceae. The accession number (MZ710458) showed 98.86% identities to that of A. ocellatum (DQ490267.1), 98.79% similarity to that of A. ocellatum (KU761581.1, KR057921.1, DQ490262.1), 98.71% similarity to that of A. ocellatum(DQ490266.1), and 97.58% similarity to that of A. ocellatum (DQ490260.1). The neighbor-joining phylogenetic tree of ITS regions of A. ocellatum exhibited two major lineages (Fig. 2). The first clade comprises the present A. ocellatum grouped with other A. ocellatum from Italy and Israel from the Mediterranean Sea.
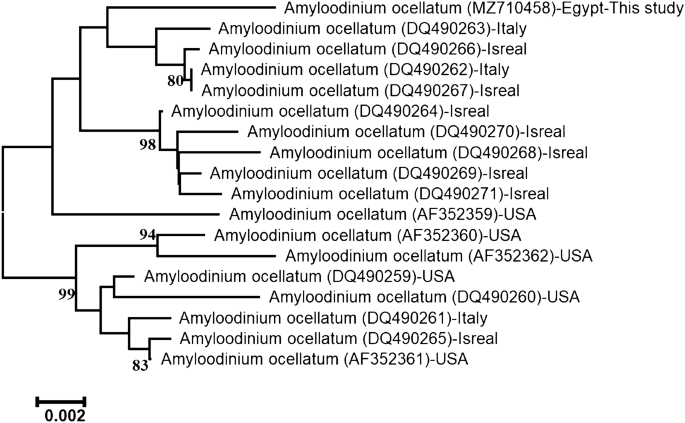
The neighbor-joining phylogenetic tree showed the comparative analysis of ITS rDNA region sequence of A. ocellatum infecting L. Carinata.
Cryptocaryon irritans
The accession number of ITS rDNA regions of this C.irritans infecting L. carinata MZ710459. The length of the sequenced ITS rDNA regions was 722-bp. Depending on its sequence alignment, the present sequence is ascribed to the species level of C. irritans and firmly embedded within the family Cryptocaryonidae. The accession number (MZ710459) showed 99.29% similarity to that of C. irritans (DQ270008.1), 99.01% similarity to that of C. irritans (DQ270009.1), 98.97% similarity to that of C. irritans (KC550300.1), and 98.75% similarity to that of C. irritans (KU761582.1, KT207810.1, AF490381.1). The neighbor-joining phylogenetic tree of the ITS rDNA gene of C. irritans showed that this sequence is strongly embedded among other C. irritans with a 100% bootstrap value (Fig. 3).

The neighbour-joining phylogenetic tree showed the comparative analysis of ITS rDNA sequence of C. irritans infecting L. carinata.
Field treatment trial
During treatment strategery, dead fish should be collected daily and buried in a hygienically based method. At the end of two weeks treatment strategery, the cumulative mortalities were dropped from 65% to about 10% after concurrent daily application of copper sulfate pentahydrate 99% and hydrogen peroxide 40% solutions into the ponds water for one week as well as application of Glutaraldehyde (15%)/Quaternary ammonium compounds 25% (QACs) combination daily for 3 successive days in the ponds water and systemic application of amprolium HCl in the fish feed for 3 successive days.Furthermore, microscopical examination of mucous skin and gills scrapings of random treated fish samples at the end of two weeks of treatment strategery showed a statistically significant decrease in the intensity of both A. ocellatum and C. irritans (Table 2).
Discussion
One of the essential requirements for the development of healthy fish is the quality of the farm water. The frequency and severity of parasitic and bacterial infections affecting fish are directly linked to the pond management practices and hygienic conditions of rearing water33,34. Poor farm management, such as overstocking, as noticed in the studied earthen ponds, promotes ectoparasites infestations34. High fish stocking levels change the balance of environmental and biological factors of the aquatic ecosystem and exacerbate protozoan infections due to higher feed inputs35. Fish ectoparasites spread rapidly in crowded aquaculture environments, leading to massive losses34.
The physicochemical water quality measures such as temperature, ammonia, DO, pH, and turbidity all significantly impact fish health and disease resistance36. The major part of these parameters in the studied farm had exceedingly deteriorated values and was supposed to predispose L. carinata fish to A. ocellatum and C. irritans protozoan infections in agreement with34. The inferior water quality measures noticed in the investigated farm could be relevant to poor management practices, including; overfeeding, inadequate replacement of water, and high fish stocking densities in the affected earthen ponds. Furthermore, excessive chicken manure addition and irresponsible fish-pond fertilization exacerbated the problem in agreement with37. According to good farm management guidelines, fish should be farmed at an optimal stocking density, and feed rates should not exceed the pond's absorption capacity38.
Water temperature (30 °C) affects the growth, establishment, and transmission of parasites'infective stages to new hosts39. High water temperature and high salinity levels recorded in the investigated farm favor numerous protozoan infestations with, including A. ocellatum and C. irritants in agreement with40. In addition, the oxygen holding capacity of the water diminishes at extreme high-water temperatures33. Furthermore, fish reared at low DO levels, like those in this study, have weaker immune systems and are more vulnerable to illness33. Poor management and low DO levels in fish-ponds enhance numerous parasitic infections33. The NH3 and NO2 levels observed were considerably exceeding the suggested optimal limits. High ammonia levels depress the immune system of fish and irritate the gills and skin, making parasitic diseases more likely33. High ammonia levels in fish-ponds may be caused by overfeeding and excess feed degradation33. Excessive turbidity in farm water may be linked to poor farm management practices such as overfeeding, overstocking, and insufficient water replenishment that increase suspended particles in the farm water37. Extreme water turbidity levels in ponds increase parasite infection risk and reduce natural food production37.
The results revealed greater phytoplankton biomass of approximately 1675 cells/ml, explaining the farm water's reddish-brown colour. These excessive algal blooms may be relevant to surplus food inputs and the high organic loads in the farm water in agreement with41. Algal blooms die-offs induce high toxic ammonia levels and oxygen depletion in farm water; both conditions are detrimental to fish and may lead to infestations. Excessive blooms also cause large pH fluctuations throughout the day, stressing and predisposing farmed fish to succumb to parasitic and bacterial infestations. Fish farmed in such low-quality water, which exactly fits the conditions in the current study, are susceptible to a variety of bacterial and parasite illnesses due to impaired immune mechanisms34,42.
Epizooties caused by A. ocellatum are well documented in the literature14,16. The prevalence of A. ocellatum infestations is influenced by various environmental factors, including temperature, and can be recorded all over the year12. Multiplication of A. ocellatum occurs at a temperature between 16 and 30 °C43. Outbreaks commonly occur at higher water temperatures (> 27 °C). The pathogenesis of this parasite is linked to the insertion of rhizoids of trophonts attachment disc into host cells, resulting in degeneration of tissues14.
The mean intensity of A. ocellatum in L. carinata tissues was 16.5 ± 2.03 in the skin and 13.18 ± 1.90 in the gills of infected fish. The findings are consistent with those of Bessat and Fadl44, who examined amyloodiniosis in two Egyptian localities, Wadi El-Natroun and El-Max, and recorded average prevalence rates of 84.86% and 39.58%, respectively, as well as average mortality rates of 42.78% and 9.86% respectively. Infections with A. ocellatum were intense (> 20 trophonts).
The mean intensity of C. irritans in the present study was 4.75 ± 1.05 in gills and 7.43 ± 1.45 in the skin of infected fish. Khalil et al.45 recorded C. irritans infestations in farmed seabream fish with a higher prevalence of 95.83% in the winter compared to 8.26%, in the summer, respectively. Infestations of C. irritans are also common in wild marine fish. Diggles and Lester46 studied C. irritans in some wild-caught marine fish. Authors recorded the highest prevalence, 100%, in Acanthopagrus australis fish with an intensity of 14.6 parasites/fish, while the lowest prevalence, 38%, was recorded in Gymnocranius audleyi with 1.9 parasites/ fish. The heaviest infection of C. irritans occurred at 17 °C. C. irritans multiplies rapidly and penetrates deeply into the integument of its host, impairing the physiological functioning of the skin and gills and therefore increasing the risk of secondary infections19. Infected fish showed more excessive mucus production and hyperplasia of epithelial cells in the gill lamellae18. The majority of the examined L. carinata were infected 90%, with 66.42% having a mixed infection and 23.57% having a single infestation of either A. ocellatum 10.71% or C. irritans 12.85%. A. ocellatum and C. irritans with varying frequency have been reported in outbreaks affecting numerous fish species worldwide12,31,42.
Morphological characteristics of detected protozoan infestations were identical to A. ocellatum and C. irritans as described in previous studies12. The molecular identification of the present parasites was performed by sequencing ITS rDNA regions flanking 18S and 28S rDNA for A. ocellatum and C. irritans. The ribosomal internal transcribed spacer (ITS) regions, the small subunit, and large subunit ribosomal DNA genes are well known as important molecular markers for identifying fish protozoans. These genes have been effectively employed to genetically categorize A. ocellatum and C. irritans in numerous studies25,26.
The current scarcity of effective authorized medications and the emergence of antiparasitic drug resistance make it difficult to control fish parasite infections in mariculture fish. Management of parasitic fish infestations requires a thorough understanding of environmental and host factors32. Therefore, there is a growing need to develop safe and effective antiparasitic drugs in aquaculture. Copper sulfate, formalin, and potassium permanganates are frequently used to treat various parasitic diseases in fish; however, these chemicals are extremely harmful and cost a lot of money29,32. The present findings showed improvement of the treated fish's health after applying the prescribed antiparasitic treatment, as shown by the lower death rate. Mortality dropped to 10% after therapy, compared to 65% before administering the recommended chemotherapeutics, indicating treatment efficiency. The intensity of protozoan infections showed a statistically significant decrease at the end of the treatment trial based on results of microscopical examinations of mucus skin and gills scrapings from treated fish after two weeks of treatment strategery.
Hydrogen peroxide is a promising chemotherapeutic that can control some fungal, bacterial, and ectoparasitic infestations affecting fish47. The Center for Veterinary Medicine of the United States Food and Drug Administration approved it to treat some infections in fish. Hydrogen peroxide was successively applied to control some parasitic infestations affecting fish, including sea lice, Lepeophtheirus salmonis on fish47, protozoan ambiphrya or the trematode Gyrodactylus spp. on rainbow trout, and A. ocellatum on the Pacific thread fish Polydactylussex filis30. The most efficient H2O2 doses for controlling protozoan infestations and monogenetic trematodes affecting fish were 170–280 mg/L administered as a static bath for 30 min. More than 280 mg/L concentrations used for 30-min exposures may be effective in controlling other parasites48.
Amprolium is a quaternized pyrimidine derivative that disrupts thiamine metabolism and prevents carbohydrate synthesis by blocking thiamine receptors. Amprolium is one of the safest anticoccidial medicines32. The ant-protozoan action of amprolium is based on blocking the thiamine transporter in Eimeria sp. meronts accordingly disrupts cell metabolism, inhibits the growth of merozoites and prevents the creation of second-generation meronts. It also slows the formation of sporozoites and affects oocyst sporulation49.
Amprolium was evaluated for its anti-protozoan action in aquaculture due to its high safety level and significant efficacy against Eimeria in chicken. In vitro, amprolium chloride was efficient against fish myxosporidium. In addition, amprolium combined with salinomycin effectively controlled Myxobolus sp. infection in some marine fish species50,51,52,53,54. Eissa et al.32 investigated the efficacy of using an amprolium-salinomycin mixture to treat heavy infestations of Myxobolus episquamalis affecting earthen pond cultured mullets in a field trial.
Conclusion
Growing healthy fish necessitates the implementation of favorable water quality measures and appropriate management practices. Poor water quality measures, including extreme temperature, low DO, excessive turbidity, high levels of nitrogenous waste products and dense algal blooms, can predispose fish to numerous protozoan infections. A. ocellatum and C. irritans are extremely harmful parasites that can harm farmed marine fish causing massive losses. Application of good management practices and efficient control methods are necessary to control protozoan infestations affecting fish. Awareness of aquaculture sustainability and environmental protection are required issues in applying antiparasitic treatments in fish farming facilities. H2O2 and amprolium have antiparasitic properties and were shown to be beneficial in reducing parasitic infestations of A. ocellatum and C. irritans protozoa in earthen-pond farmed L. carinata.
Data availability
All data and materials are available within the article.
References
Shaalan, M., El-Mahdy, M., Saleh, M. & El-Matbouli, M. Aquaculture in Egypt: Insights on the current trends and future perspectives for sustainable development. Rev. Fisheries Sci. Aquacult. https://doi.org/10.1080/23308249.2017.1358696 (2017).
Sadek, S. Sea bream culture in Egypt; status, constraints and potential. Fish Physiol. Biochem. 22, 171–178 (2000).
GAFRD. General authority for fish resources development. in Fish Statistics Year Book. (Ministry of Agriculture and Land Reclamation, 2018).
Kaleem, O. & Sabi, A. S. Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquacult. Fisheries. https://doi.org/10.1016/j.aaf.2020.07.017 (2020).
Gonzalez Castro, M., Heras, S., Cousseau, M. B. & Roldan, M. I. Assessing species validity of Mugil platanus Günther, 1880 in relation to Mugil cephalus Linnaeus, 1758 (Actino pterygii). Ital. J. Zool. 75, 319–325 (2008).
Sadek, S. Culture of Mugilidae in Egypt. in Biology, Ecology and Culture of Grey Mullets (Mugilidae), 501–513. (Taylor & Francis Group, LLC, 2016). https://doi.org/10.1201/b19927-21.
Thomson, J. M. The Mugilidae of the world. Mem. Qld. Mus. 41(3), 457–562 (1997).
Mehanna, S. F. Population dynamics of keeled mullet, Liza carinata and golden grey mullet, Liza aurata at the Bitter Lakes, Egypt. Bull. Nat. Inst. Oceanogr. Fish. ARE. 30, 315–321 (2004).
Crosetti, D. & Blaber, S. J. (eds) Biology, Ecology and Culture of Grey Mullets (Mugilidae) 124–125 (CRC Press, 2015).
Buchmann, K. Impact and control of protozoan parasites in mari-cultured fishes. Parasitology 142, 168–177. https://doi.org/10.1017/S003118201300005X (2015).
Noga, E. J. Fish Disease: Diagnosis and Treatment 367 (Wiley-Blackwell, 2010).
Saraiva, A., Jeronimo, D. & Cruz, C. Amyloodinium ocellatum (Chromalveolata:dinoflagellata) in farmed turbot. Aquaculture 320, 34–36. https://doi.org/10.1016/j.aquaculture.2011.07.034 (2011).
Kumar, R. P. et al. Amyloodinium ocellatum infestation in the broodstock of silver pompano Trachinotus blochii (Lacepede, 1801) and its therapeutic control, Indian. J. Fish. 62, 131–134 (2015).
Paperna, I. Amyloodinium ocellatum (Brown, 1931) (Dinoflagellida) infestations in cultured marine fish at Eilat, Red Sea: Epizootiology and pathology. J. Fish. Dis. 3, 363–372. https://doi.org/10.1111/j.1365-2761.1980.tb00421.x (1980).
Paperna, I. Reproduction cycle and tolerance to...
Comments
Post a Comment