Diversity of MHC IIB genes and parasitism in hybrids of evolutionarily divergent cyprinoid species indicate heterosis advantage | Scientific Reports - Nature.com
Abstract
The genes of the major histocompatibility complex (MHC) are an essential component of the vertebrate immune system and MHC genotypes may determine individual susceptibility to parasite infection. In the wild, selection that favors MHC variability can create situations in which interspecies hybrids experience a survival advantage. In a wild system of two naturally hybridizing leuciscid fish, we assessed MHC IIB genetic variability and its potential relationships to hosts' ectoparasite communities. High proportions of MHC alleles and parasites were species-specific. Strong positive selection at specific MHC codons was detected in both species and hybrids. MHC allele expression in hybrids was slightly biased towards the maternal species. Controlling for a strong seasonal effect on parasite communities, we found no clear associations between host-specific parasites and MHC alleles or MHC supertypes. Hybrids shared more MHC alleles with the more MHC-diverse parental species, but expressed intermediate numbers of MHC alleles and positively selected sites. Hybrids carried significantly fewer ectoparasites than either parent species, suggesting a hybrid advantage via potential heterosis.
Introduction
Reciprocal interspecific hybrids are often characterized by high vigour resulting from heterosis1,2. Hybrid advantage usually reflects the superiority of F1 hybrids over one or both parents for traits related to development, growth, maintenance, and resistance to environmental factors and diseases (e.g.3,4,5). In line with the hybrid advantage hypothesis, fish hybrids often exhibit higher potential to survive, faster growth, and better condition status when compared to parental species (e.g.6,7,8). In spite of the high frequency of hybridization in fish and especially in highly diversified Cyprinoidei9, the potential of immune resistance/susceptibility has not been comprehensively studied in fish hybrids coexisting in natural habitats with their parental species.
In hybrids of wild living vertebrates, selection may favor the variability of immune genes associated with high resistance to parasites, and potentially interspecies hybrids may through the variability of immune genes exhibit a survival advantage over parents. The major histocompatibility complex (MHC) includes the functional immune genes recognized as an essential component of the vertebrate adaptive immune system (although MHC is absent in jawless fish). Thus, MHC may represent appropriate candidate of immune genes when investigating hybrid heterosis.
A characteristic feature of MHC genes is trans-species polymorphism, i.e. the passage of allelic lineages from ancestral to descendant species. This polymorphism was previously documented in cyprinoids10,11. In teleost fish (as well as in other jawed vertebrates), two groups of classical MHC genes—class I and class II have been recognized and described. Whilst MHC class I molecules predominantly bind peptides derived from intracellular pathogens (viruses and bacteria), MHC class II molecules bind peptides derived from extracellular pathogens (especially macroparasites)12,13. MHC molecules play a central role in parasite recognition and elimination. The binding between MHC molecules and foreign peptides derived from parasites is realized by a small number of amino acid residues of the peptide-binding regions (PBR)13,14,15. Therefore, parasite-mediated selection is considered as one of the main drivers of the evolution and maintenance of high MHC polymorphism14,16,17,18,19, and thus, the MHC provides a genetic basis for the adaptation of vertebrate hosts to coevolving parasites. Using a theoretical model, an intermediate number of MHC alleles was postulated as the optimal MHC for an individual20. Based on this model, the maximum number of MHC alleles is disadvantageous because it results in the presentation of more self-peptides with the subsequent elimination of self-reactive T-cells in individuals. In the study of three-spined stickleback, an intermediate number of MHC IIB alleles was associated with minimal parasite load at the individual level21. However, the super-optimal individual diversity of MHC genes in hybrids was hypothesized, with the expectation that hybrids with super-optimal MHC diversity should suffer more from parasites22. This mechanism should be very effective in selecting against interspecies hybrids. Concerning wild-living vertebrates, the genetic variation in MHC genes between hybridizing species was studied, and adaptive MHC introgression was documented, especially in newts23,24,25. The mechanisms generating MHC diversity in hybrid zones were also investigated in fishes26 focusing on hybrid zones of native and endemic Parachondrostoma toxostoma and invasive Chondrostoma nasus (Leuciscidae) in Southern France. Bidirectional gene flow for MHC IIB genes was shown. The authors reported the expression of an intermediate number of MHC IIB alleles in hybrids of the first generation (F1 hybrids), representing their potential advantage; however, hybrids expressed a higher proportion of MHC genes of more genetically variable species, i.e. native P. toxostoma. Higher MHC similarity between genetically more MHC-variable species and hybrids was proposed to explain the low susceptibility of native P. toxostoma and hybrids to ectoparasitic monogeneans widely infecting non-native and genetically less diverse C. nasus26. Innate and adaptive immune-relevant factors can be transferred maternally, some of them functioning in the defense of fish larvae against pathogens27. However, paternal effects have also been reported for innate immunity28. This may evoke the question of whether the direction of genetic introgression affects hybrid immunocompetence and susceptibility to parental species-specific parasites.
Evolutionary divergence is an important mechanism affecting genetic incompatibilities between species and determining the successfulness of hybrids. The hybridization between common bream (Abramis brama) (henceforth, 'bream') and roach (Rutilus rutilus), two evolutionarily divergent leuciscid species, has been widely documented29,30,31,32. Whilst a strong bias toward hybrids with bream maternal ancestry was documented in some regions32,33,34, similar proportions of hybrids with bream and roach maternal inheritance were documented in other regions35. Hybrids of bream and roach in natural habitats are primarily produced as F1 crosses of parental species, and the presence of post-F1 hybrids is negligible31,32,33 indicating that F1 hybrids experience fitness disadvantages when compared with pure species. In some regions, all hybrids of bream and roach sampled in natural habitats are identified as solely F1 crosses34,35. F1 hybrids of these leuciscid species express intermediate phenotypic and ecological traits32,34, utilization of a broader trophic spectrum and high tolerance to fluctuations of food supply compared to parental species31,36. Hybrid advantage was documented in terms of high survival at early developmental stages37, fast growth38, and low susceptibility to parasites that are host-specific to bream or host-specific to roach35.
The aim of the present study was to analyze the variability of expressed MHC genes in natural populations of two coexisting and evolutionarily divergent leuciscid species, A. brama and R. rutilus, and their reciprocal hybrids (F1 generation). In accordance with previous studies in hybrid zones of cyprinoids26, we hypothesized that the intermediate variability of MHC genes in hybrids, measured by the number of DAB alleles, is associated with low parasite load (lower parasite load in F1 hybrids of bream x roach when compared to parental species was previously shown35). We also hypothesized some similarity in MHC profiles between hybrids and each of the parental species, on the basis that hybrids may harbor the specific parasites of both parental species in cyprinoid hosts35,39. Finally, based on the prediction that MHC is involved in recognition of parasites, we hypothesized the potential associations between specific MHC alleles (present only in one parental species and potentially in hybrids) and specific parasite species (also present only in one parental species and potentially in hybrids). We also investigated potential functional associations between MHC (using MHC supertypes) and parasites.
Results
MHC diversity and positive selection
The amplification of cDNA representing expressed DAB genes was successful in 94% of analyzed specimens (Table 1). The overall number of expressed DAB1 alleles was lower than the number of expressed DAB3 alleles in all three groups of fish. Numbers of specimens for which the expression of only the alleles of DAB1 genes, only the alleles of DAB3 genes and the expression of the alleles of both DAB1 and DAB3 genes were reported, are shown in Table 1. Most roach specimens expressed the alleles of both DAB1 and DAB3 genes, whilst most bream and hybrids expressed the alleles of DAB3 genes only.
The total number of DAB alleles per fish group, total number of private alleles, and number of alleles per individual are shown in Table 2. Roach were more polymorphic than bream, and hybrids were intermediate. However, the overall number of DAB1 alleles was similar in bream and hybrids and was lower when compared to roach (Table 2). Hybrids shared five DAB1 alleles with roach and only one DAB1 allele with bream. A single DAB1 allele was shared among all three groups of fish. Hybrids shared 13 DAB3 alleles with roach and 5 DAB3 alleles with bream; another five DAB3 alleles were shared among all three groups of fish.
Different alleles were reported as the most frequent for bream and roach populations (Fig. 1). Bream frequently expressed two specific alleles (found solely in bream and hybrids) and three other alleles shared with hybrids and roach. Roach most frequently expressed four specific alleles. Other alleles in bream and roach were present at frequencies ≤ 6%. Hybrids most frequently expressed the common alleles of parental species (two roach-specific alleles and four alleles frequently reported in bream). Other alleles in hybrids were present at frequencies < 6%. Hybrid-specific alleles were present at frequencies < 3.4%.The hybrids with bream in maternal position and the hybrids with roach in maternal position expressed some of the common alleles in different frequency (Supplement S1).
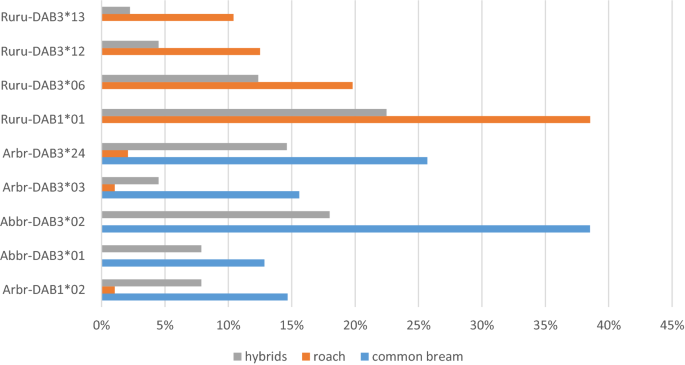
The frequencies of the most common DAB alleles in common bream, roach and their F1 hybrids. Note that only the frequencies of the most frequent alleles for common bream (5 alleles) and roach (4 alleles) are shown.
The mean number of DAB alleles per hybrid individual, as well as the maximum number of alleles in hybrid individuals, was intermediate between those of bream and roach (Table 2). A significant difference in the number of DAB1 alleles was found among fish groups (Kruskal–Wallis H-test, p < 0.001). Hybrids expressed an intermediate number of DAB1 alleles between bream and roach; multiple comparisons revealed significant differences between bream and roach (p < 0.001) and between hybrids and each of the parental species (p < 0.05). The Kruskal–Wallis H-test also revealed a significant difference in the total number of DAB3 alleles among bream, roach, and hybrids (p = 0.015). Even though the hybrids tended to express the intermediate number of DAB3 alleles, multiple comparisons revealed the significant difference only between bream and roach (p = 0.036).
Phylogenetic reconstruction (Fig. 2) showed that DAB alleles clustered in two main lineages, i.e. the lineage of DAB1 alleles and the lineage of DAB3 alleles. Even if some clusters tended to include mostly roach alleles and others mostly bream alleles, many clusters included the alleles of both species, which supported trans-species polymorphism. A few alleles shared by both species and their hybrids were situated randomly within a phylogenetic tree.
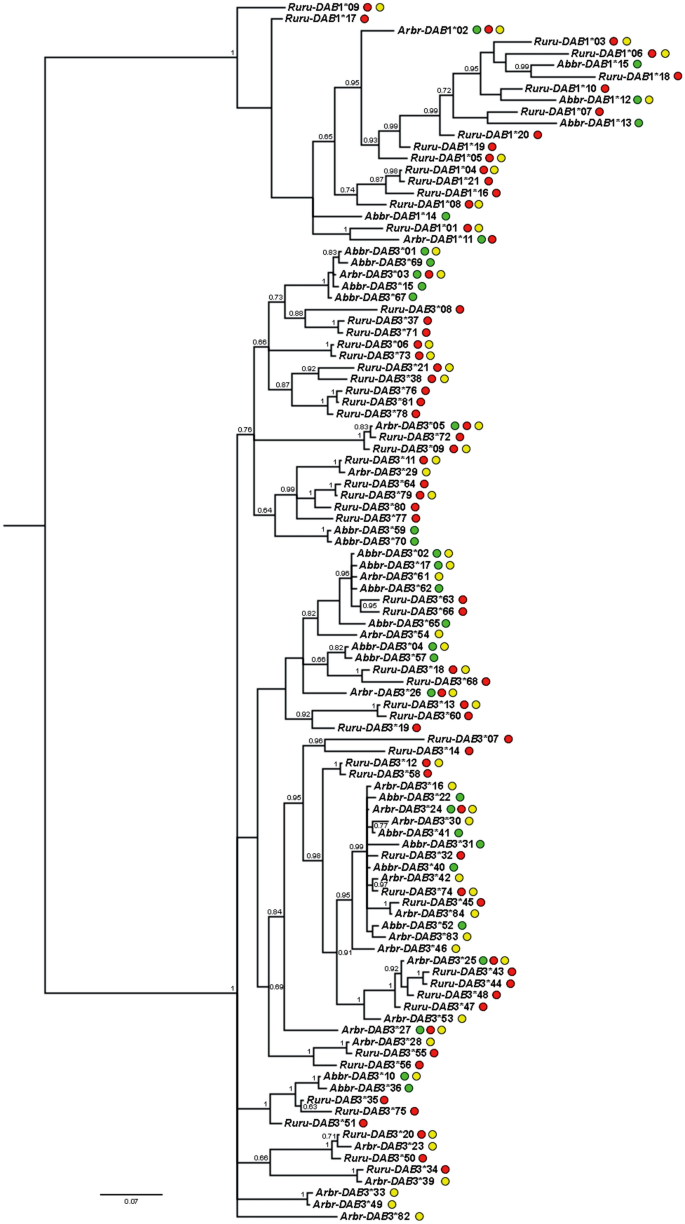
The Bayesian inference tree including DAB1 and DAB3 alleles identified in A. brama, R. rutilus and their hybrids. Numbers along branches represent posterior probabilities (> 0.60) resulting from BI. Green dots indicate the alleles present in A. brama, red dots indicate the alleles present in R. rutilus, and yellow dots indicate the alleles present in hybrids. DAB alleles were abbreviated as follows: Abbr present solely in A. brama or both A. brama and hybrids, Ruru present solely in R. rutilus or both R. rutilus and hybrids, Arbr alleles present in both parental species and hybrids, or alternatively present solely in hybrids.
The likelihood ratio statistic comparing the two models—one not incorporating selection and one incorporating selection (M1a versus M2a, M0 versus M3, M7 versus M8)—indicated that the models that accounted for the sites under selection (i.e. M2a, M3 and M8) fit the data significantly better (p < 0.001) than simpler models that did not allow for selection (i.e. M1a, M0 and M7), which indicates a signal of positive selection at specific sites in DAB sequences. Log-likelihood values and parameter estimates under random-site models are shown in Supplement S2. On the basis of BEB analysis, 24 and 33 PSS in bream were identified using the M2a and M8 models respectively, whilst 25 sites using the M2a model and 26 sites using the M8 model were identified under positive selection in roach. The Bayes identification of sites under positive selection calculated using the M8 model is included in Fig. 3. The same 24 PSS under the M8 model were identified in both species. In addition, next 9 codons only in bream and 2 codons only in roach were identified under positive selection. The pattern of PSS distribution in hybrids was intermediate between bream and roach. The 24 PSS shared by bream and roach were also identified in hybrids. In addition, one codon under positive selection was shared by both bream and hybrids, and one codon under positive selection was shared by roach and hybrids. A single codon was identified under positive selection solely in hybrids.
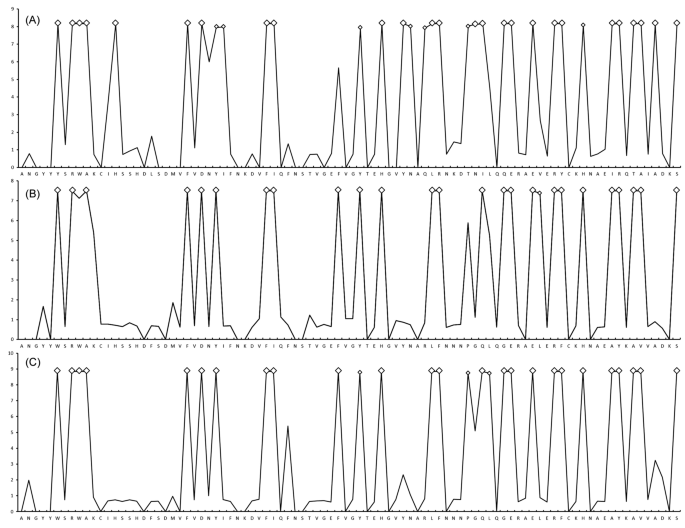
Approximate posterior means of ω calculated as the weighted average of ω over the 11 site classes and weighted by the posterior probabilities under the M8 site-model are shown for DAB sequence variants for (A) A. brama, (B) R. rutilus, and (C) their respective hybrids. Sites inferred to be under positive selection at the 99% level are indicated by large white squares and those at the 95% level are indicated by small white squares.
Associations between MHC genes and parasites
Parasite data for fish analysed for MHC diversity are included in Table 3. No significant covariance between MHC alleles and parasite species data or parasite groups data was found using COIA for each of bream, roach, and hybrids separately (p > 0.05). No significant covariance between MHC alleles and parasite groups was found when including all fish groups into analysis (p > 0.05) (Supplement S3). Similarly, no significant covariance between MHC supertypes and parasite groups data (Supplement S4) was found when including all fish in the analyses or when analyzing common bream, roach or hybrids separately (p > 0.05). When COIA was performed including MHC alleles and all fish groups (bream, roach, and hybrids), the DAB alleles and metazoan parasite species exhibited significant covariance in the COIA model (RV = 0.16, p < 0.003). The two first axes of the COIA (F1 and F2) accounted for 75.05% of the total variance shared between the two matrices (F1 65.99% and F2 9.06%). Comparison between the distribution of parasitological and genetic variables on the COIA factor maps revealed species-specific associations (Fig. 4). Correspondence analysis row scores on F1 separated bream and roach individuals on the basis of their specific MHC alleles and host-specific parasite species (Supplement S5).
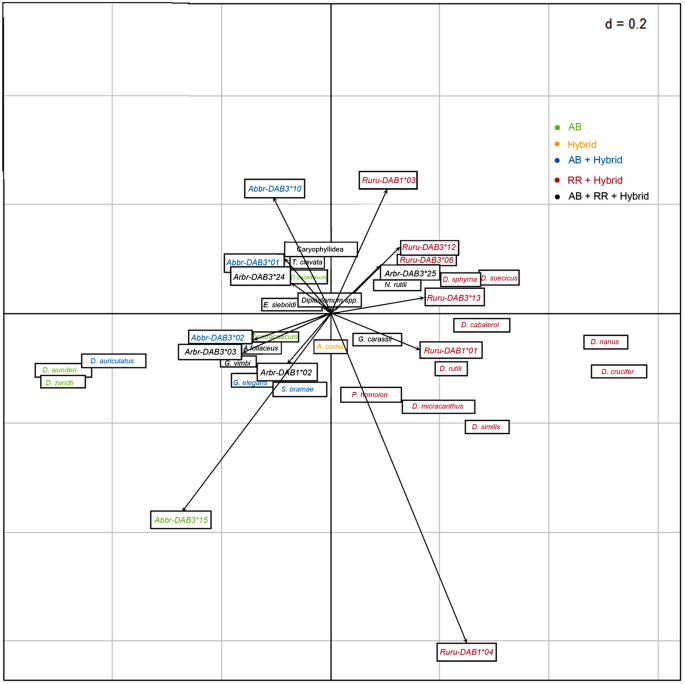
Co-inertia analysis (COIA) of the MHC genetic variation (MHC alleles data) and metazoan parasite species of common bream, roach and hybrids. Color labelling of DAB alleles and parasites corresponds to their presence in different fish groups or shared groups.
The total number of alleles, number of DAB1 alleles, or number of DAB3 alleles appeared to have no or very limited effect on parasite abundance/prevalence, as this effect was significant only in three out of 26 GLMM models testing the effects of season, fish group and number of alleles on parasite load. The effects of season and species were more pronounced, being present in the final models for most of the taxonomical groups (Table 4, see Fig. 5 for ectoparasites). Generally, fish were more parasitized in spring than in autumn, both in terms of prevalence, abundance and richness. Whilst roach harboured higher Dactylogyrus species richness when compared to bream and hybrids, bream reached higher total parasite abundance, ectoparasite abundance, monogenean abundance, and Dactylogyrus abundance. Bream and hybrids reached a higher abundance of Crustacea when compared to roach, and hybrids themselves also reached a higher abundance of Digenea when compared to bream and roach. For the significance of the abovementioned differences see Supplement S6.
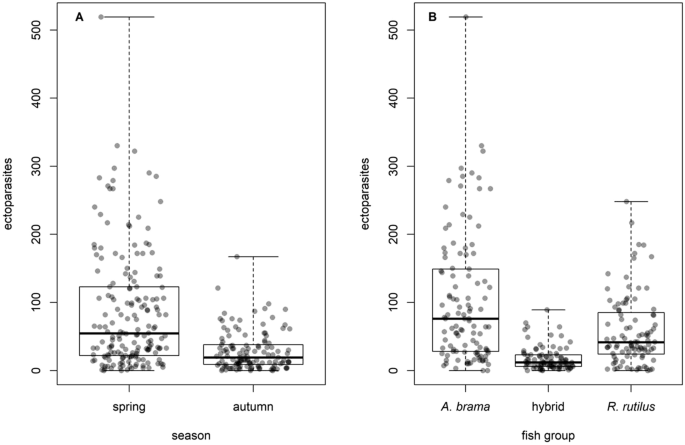
Effects of season (A) and fish group (B) on ectoparasite abundance.
The effects of numbers of DAB1 or DAB3 alleles on parasites were rare, relatively weak, and inconsistent (Table 4, Supplement S6). In the case of endoparasites, a significant interaction between fish group and number of DAB1 alleles resulted from the increase in abundance with the number of DAB1 alleles in hybrids, with no such trend occurring in roach or bream (Fig. 6). In the case of Gyrodactylus, there was a significant effect of the interaction between species and number of DAB3 alleles, with Gyrodactylus prevalence decreasing with the number of DAB3 alleles in roach; no such trend was observed in bream or hybrids. The prevalence of Nematoda was affected by fish group (bream was most highly infected, while no infection was found in roach) and a decrease in the number of DAB1 alleles. However, the total prevalence of Nematoda infection was very low (see Table 3).
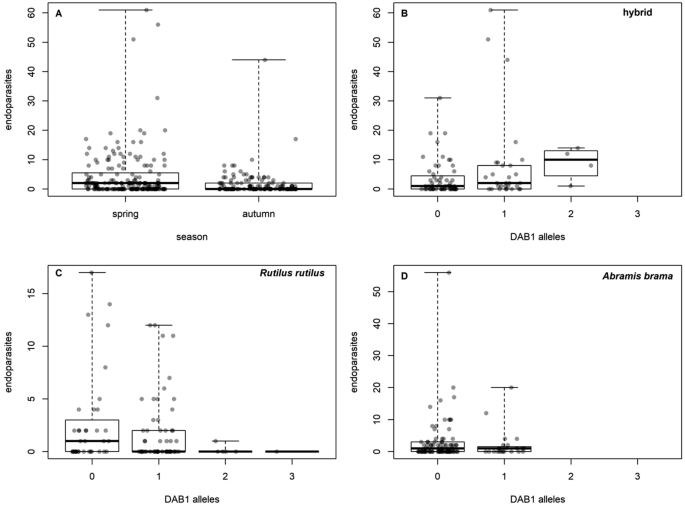
Effect of season on endoparasite abundance (A) and effect of the number of DAB1 alleles on endoparasite abudance in hybrids (B), R. rutilus (C) and A. brama (D).
When analyses were performed separately for bream, roach, and hybrids in order to examine the effects of the most common DAB alleles on parasite load, GLMM revealed a strong effect of season on parasite load but only limited effects of DAB alleles (Table 5). In roach, the presence of the Ruru-DAB3*06 allele had a positive association with Argulus foliaceus prevalence and a negative association with D. similis abundance, while the presence of the Ruru-DAB1*01 allele had a negative association with D. caballeroi abundance and a positive association with D. micracanthus prevalence. In bream, the presence of the Arbr-DAB3*03 allele had a negative association with Ergasilus sieboldi abundance and a positive association with G. vimbi prevalence. No other significant associations between DAB alleles and parasite abundance or prevalence were observed (Table 5).
When tested for the spring season only (Supplement S7), the GLMM test confirmed the signficance of Ruru-DAB3*06 effects on A. foliaceus and D. similis and a Ruru-DAB1*01 effect on D. caballeroi in roach, with other associations becoming non-significant. In contrast, total parasite abundance, ectoparasite abundance, and the abundance of A. foliaceus and E. sieboldi (the two crustaceans comprising 49% of spring ectoparasites and 37% of all spring parasites) became significantly positively associated with the presence of the Ruru-DAB1*01 allele in hybrids when tested for spring samples only.
When analyses were performed separately for bream, roach, and hybrids in order to examine the effects of the most common MHC supertypes on parasite load, GLMM revealed a strong effect of season on parasite load but only limited effects of MHC supertypes (Table 6). In roach, the presence of supertype E had a positive association with A. foliaceus prevalence and negative association with D. similis abundance. In addition, the presence of supertype A had positive association with D. caballeroi, D. similis and D. micracanthus. Finally, the presence of supertype C had negative association with Tylodelphys clavata. In hybrids, supertype A was associated with total parasite abundance, ectoparasite abundance, endoparasite abundance and abundance of T. clavata. In contrast, supertype E was negatively associated with Diplostomum spp. and D. micracanthus, but positively associated with G. elegans prevalence. In bream, supertype E was negatively associated with E. sieboldi prevalence and positively associated with G. vimbi prevalence. The presence of supertype A was negatively associated with D. zandti abundance. When tested for the spring season only (Supplement S8), very similar associations between MHC supertypes and parasite load were found. Four associations reported between MHC supertypes and parasite load for whole sample were also found using spring season only. Moreover, positive association between supertype C and A. foliaceus abundance, and negative association between supertype E and D. micracanthus abundance were found. In hybrids, five out of seven associations were revealed by both analyses i.e. using whole sample and using spring only. In bream, only the association between supertype E and G. vimbi prevalence was the same in both analyses. Using spring sample, two supertypes—D and E were positively associated with D. zandti abundance.
Discussion
Hybridization is a common phenomenon in fish9,40; however, prior to this study, the variability in functional immune genes in naturally distributed fish hybrids coexisting in the same natural habitats with their parental species was only rarely investigated26. Most analyses of functional immune genes come from experimental studies, e.g. field mesocosm experiments using Gasterosteus aculeatus41,42. The hybridization between evolutionarily divergent bream and roach is one of the best documented cases of hybridization in wild living cyprinoids. In our study, we hypothesized that MHC diversity in F1 hybrids may reflect the hybrid advantage of being less parasitized when compared to their parental species due to the heterosis effect. A lower level of parasite infection in F1 hybrids of bream and roach was documented35. As MHC molecules play a central role in parasite recognition and elimination, following previous studies documenting potential associations between MHC diversity or MHC genotype and metazoan parasites in fish39,43,44, we investigated the extent of similarities in MHC profiles between hybrids and each of the parental species. In addition, we examined the potential associations between the most common MHC alleles or MHC supertypes and parasite load.
In our study, we are dealing with the bidirectional hybridization of evolutionarily divergent cyprinoids exhibiting divergent specific metazoan parasitofauna (with a large proportion of host-specific gill and skin monogeneans). Our finding that hybridization of bream and roach was almost perfectly bidirectional is surprising, given previous research in other European countries suggesting that territorial behavior of bream males reduces the opportunity for mating with roach females32,45.
We showed that bream and roach tended to express highly divergent MHC profiles, and only a few alleles were shared between the two cyprinoid species as a result of the trans-species evolution widely documented in MHC of fish10,46,47. Phylogenetic reconstruction of MHC alleles seems to indicate that genetic introgression may operate in the hybridizing system studied. The previous study in cyprinoids indicated that MHC diversity in populations of hybridzing species is shaped by both trans-species and genetic introgression26. However, Wegner and Eizaguirre24 highlighted that it is very difficult to disentangle the underlying signatures of trans-species polymorphism from recent introgression and proposed to investigate recombination rates in the chromosomes carrying the MHC genes. According to the allelic profile of MHC genes, population variability was substantially different in bream and roach in the investigated water reservoir. The origin of this variability is unknown as we have no data on the possible establishment of these two cyprinoid species in the Hamry reservoir by human activity or by their own potential invasion of this artificial reservoir from the surrounding rivers. During our investigations, bream was more abundant than roach. The higher MHC polymorphism reported in roach could potentially indicate the multiple origin of roach entering into the reservoir and/or different times of colonization of two species (more recent colonization by bream). To resolve the origin of fish populations, surrounding fish populations connected with the Hamry reservoir should be studied using multiple genetic markers; however, there are no data concerning the potential sources of fish introduction by human activity in the locality of interest. Our former hypothesis may be supported by the interpopulation study based on the MHC study of native Parachondrostoma toxostoma and invasive Chondrostoma nasus in their hybrid zones in Southern France26, where higher polymorphism at population level was reported in native (i.e. more established) species than in the invasive one (i.e. natural colonizer). In contrast to the study by Šimková et al.26, we found that higher MHC polymorphism in roach is associated with a higher number of roach-specific alleles (more than twice the number in roach when compared to bream).
For each of the cyprinoid species investigated in the present study, several specific alleles were reported at a high frequency and these specific alleles were also reported at high frequencies in hybrids of the F1 generation. Previously, the data on metazoan parasites in these naturally co-occurring bream, roach, and F1 hybrids were analyzed, and the presence of all parental species-specific ectoparasites (mainly gill and fin monogeneans) in hybrids was demonstrated35 (shown also in Table 3); however, parental species-specific parasites reached lower levels of infection in hybrids compared to their infection levels in associated bream or roach hosts. This may indicate that the presence of specific MHC alleles in hybrid genomes determines the presence of host-specific parasites or, alternatively, if MHC plays a role in host-parasite coadaptation, that a lack of co-adapted MHC alleles determines a low degree of hybrid susceptibility to host-specific parasites of both species. Previous studies have suggested that direct hybridization in cyprinoids (resulting in the F1 generation of hybrids) likely precludes high intensities of parental species-specific parasites in non-coadapted host genomes11,26,35, which may potentially represent one of the advantages of hybrid heterosis for the F1 generation. However, this hypothesis should be carefully reinvestigated using hybrid genomes with expected higher genetic incompatibilities associated with hybrid breakdown, i.e. backcrossed and F2 generations of hybrids.
In the present study, some hybrid-specific MHC allelic variants were observed, a pattern which was previously documented also for hybrids of Parachondrostoma toxostoma and Chondrostoma nasus26. The majority of these hybrid-specific alleles originated from roach, as indicated by phylogenetic reconstruction, a species with higher MHC diversity at the population and individual levels. In accordance with Šimková et al.26, we found that the MHC variability in hybrids was intermediate between parental species. It was hypothesized that the hybridization of highly divergent species with a likely divergent MHC repertoire increased the number of MHC alleles in hybrids22. A high number of MHC variants then leads to the recognition of a high number of foreign antigens by T-cells but is also responsible for the elimination of self-derived peptides (shown for MHC I in the bank vole (Myodes glareolus)48). In accordance with this hypothesis, high parasite infection should be observed in hybrids, which is not, however, the case of cyprinoid hybrids of the F1 generation11,35,49.
In this study, we demonstrated some maternal effect on the MHC expression profile of hybrids. The expression of parental specific MHC alleles was slightly biased in accordance with the maternal origin of hybrids, i.e. hybrids with roach maternal origin expressed a higher number of DAB3 alleles recognized in roach. The most common allele of roach, Ruru-DAB1*01, and the most common allele of bream, Abbr-DAB3*02, were present in F1 hybrids with roach or bream in the maternal position. However, only hybrids with bream in the maternal position expressed one of the common alleles of bream, i.e. Arbr-DAB1*02. Hybrids with roach maternal origin expressed one of the specific alleles of roach, i.e. Ruru-DAB3*06, at a higher frequency than hybrids with bream maternal origin. The expression of some MHC alleles specific to one or the other parental species may be potentially related to the different levels of infection by some metazoan parasite species (digeneans and crustaceans) previously shown for bream and roach35.
We showed that hybrids express an intermediate number of positively selected sites (potential reflecting optimal MHC), and finally, we revealed that hybrids carried lower levels of metazoan parasite infection when compared to parental species (see also35). This finding could be interpreted as representing an advantage for hybrids over their parental species. However, it should be also taken into account that hybrids less infected by parental species-specific parasites (monogeneans representing the dominant part of parasite communities) reached higher levels of infection by generalist parasites (especially by digeneans and partially by crustaceans).
The spectrum of DAB alleles expressed in hybrids included approximately 18% of the alleles that were not shared with bream and roach specimens. Crossover between non-sister chromatids during meiosis may potentially represent the molecular mechanism leading to generation of new MHC variants in hybrids. Previously, Šimková et al.26 suggested that low sample size may generate hybrid-specific alleles in the MHC data set. However, it does not seem to be a case in our study as about 100 specimens for each of cyprinoid species and their hybrids were investigated (alternatively even more higher sample size is necessary when investigating MHC genes with high level of polymorphism). Mathematical simulations suggest that frequency-dependent selection resulting from Red Queen dynamics and promoting the advantage conferred by novel alleles may be considered a more important mechanism driving MHC diversity than the mechanism of heterozygote advantage50,51. This was evidenced in the study of guppies (Poecilia reticulata) experimentally infected by the common monogenean ectoparasite Gyrodactylus turnbulli52. In the study of guppies, it was shown that host specimens carrying new MHC variants experienced a 35–37% reduction in the intensity of parasite infection, but that the number of MHC variants carried by an individual was not a significant predictor of parasite load52. However, the populations of three-spined stickleback exposed to a wider range of parasites tended to be more diverse in MHC IIB genes, and it was proposed that high MHC diversity in wild species is more likely a result of multiple host gene and parasite coevolution21. Our results agree with Philips et al.52 as we did not identify clear evidence supporting that the number of MHC alleles is a predictor of parasite load, i.e. parasite species richness and especially the abundance of common ectoparasite groups were not associated with the number of MHC alleles. However, it should be also taken into account that the links between MHC and parasites may be underpowered in statistical analyses as previously suggested by Gaigher et al.53 when studying a link between MHC variation and immunocompetence in wild populations.
Our study does not indicate that DAB genes represent the functional immune genes involved in the long-termed coadaptation of host-specific monogeneans and their associated hosts, at least when considering the presence of the most commonly expressed MHC alleles or MHC supertypes (grouping MHC alleles based on functional similarities). We performed COIA analyses using different data sets. No associations between parasites and MHC supertypes were evidenced. Using parasite group data and MHC allele data, we found no significant correlation. However, presence of Abbr-DAB3*10 was weakly associated with Digenea in bream and hybrids, which are both more susceptible to digenean infection when compared to roach. Another potential association was found between one of the most common alleles Abbr-DAB3*1 reported in bream and hybrids and infection by Crustacea (Supplement S3). Even though the correlation between parasite species data and MHC allele data was revealed by COIA in our study, the output of this analysis only clearly demonstrated the strong differentiation of two divergent cyprinoid species based on their specific MHC allelic profiles and the presence of host-specific parasites. Primarily, we can interpret the associations between MHC genes and parasites by two non-mutually exclusive hypotheses: (1) MHC genes may represent the candidate immune genes reflecting long-term host-parasite coadaptation12,54—in such a case the species-specific MHC alleles should be associated with the presence of host-specific parasite species, and/or (2) MHC is under local adaptation, i.e. the expression of the most common MHC alleles reflects the increasing local intensity of infection by the most common parasite as a result of negative frequency-dependent selection between the most common host and parasite genotypes55, as shown for fish49.
However, using a univariate approach based on abundance or prevalence parasite data, only a few associations were revealed between the most common DAB alleles and the most common parasite groups or parasite species for roach or bream, although it was possible that these associations were generated randomly because of the high number of statistical tests performed. More specifically, Ruru-DAB1*01 and Ruru-DAB3*06 alleles in roach may be involved in associations with more abundant species (two roach-specific Dactylogyrus species and generalist Argulus foliaceus), and the pres...
Comments
Post a Comment