Oral Colonization by Entamoeba gingivalis and Trichomonas tenax: A PCR-Based Study in Health, Gingivitis, and ... - Frontiers
Introduction
The role of microbiota in health and disease among humans has recently been demonstrated using molecular methods (Cho and Blaser, 2012; Siqueira and Rocas, 2017; Tsuji et al., 2018). The oral microbiota was no exception, and its disruption has been linked to a various range of oral diseases including gingivitis and periodontitis (Lourenco et al., 2014; Lamont et al., 2018; Santi-Rocca, 2020).
The complex interactions of different resident microbes that result in an equilibrium to maintain the healthy state of the oral cavity is termed oral eubiosis (Lamont et al., 2018). In contrast to eubiosis, the disruption in oral microbiota's homeostatic state is referred to as oral dysbiosis (Darveau, 2010; Lourenco et al., 2014; Kinane et al., 2017).
Periodontal disease represents a state of chronic inflammation in gingiva, bone and supporting ligaments, with gingivitis and periodontitis as the most common presentations (Di Benedetto et al., 2013; Kononen et al., 2019). The physiologic healthy state of gingiva can be defined as the total absence or minimal levels of clinical inflammation of the periodontium with normal support (no loss affecting attachment or bone) (Caton et al., 2018; Lang and Bartold, 2018). The identification of plaque‐induced gingivitis relies on the presence of bleeding on probing with an intact periodontium and/or visible inflammation; and this condition can be reversed back to a healthy state if managed properly (Caton et al., 2018; Trombelli et al., 2018). Periodontitis results in the destruction of periodontal ligament, cementum and alveolar bone, as well as migration of the long junctional epithelium. The inflammation and microbiota of periodontitis can be controlled; however, the tissues are not healed back to their initial volume, organization, and shape (Caton et al., 2018). Thus, continual maintenance of good oral hygiene is a necessity in such case (American Academy of Periodontology, 2011; Zimmermann et al., 2015; Lertpimonchai et al., 2017).
Periodontal disease is considered among the most common diseases affecting all age groups with predilection for the elderly (Kinane et al., 2017; Tonetti et al., 2017). As of 2010, the prevalence of periodontitis was 47% among adults aged 30 and above in the United States, while the global prevalence of severe periodontitis was 11%, with higher estimates for gingivitis (Eke et al., 2012; Jin et al., 2016; Nazir, 2017). In addition, periodontitis is considered an important cause of tooth loss in older adults, which adversely affects the quality of life among this group (Griffin et al., 2012). A recent study estimated the prevalence of periodontitis among dentate US adults aged 30 years or more at 42%, with 7.8% having severe form of the disease (Eke et al., 2020).
The underlying etiology and pathogenesis of periodontal disease has been linked to microbial dysbiosis (Mira et al., 2017; Sudhakara et al., 2018). However, the exact specific roles of different microbes in the dental plaque that could lead to the development of periodontal disease remains an enigma (Darveau, 2010; Hajishengallis, 2015). In addition, the initiating factors for microbial dysbiosis in the oral cavity remains unclear and deciphering such factors is the subject for ongoing research (Lamont et al., 2018).
Several microbiological patterns can be identified in periodontal diseases, in association with some specific pathophysiological traits, sustaining that the diversity in periodontitis is not limited to the variety of the current consensual classification of clinical presentations (Offenbacher et al., 2016). However, the presence of inflammation-related bone destruction is a common defining characteristic of periodontitis (Cekici et al., 2014). Thus, deep understanding of the inflammatory and immunologic processes observed in periodontal disease is of prime importance in any attempt for management of such a highly prevalent disease (Kononen et al., 2019).
Several risk factors have been linked to an increased incidence of periodontal disease; and these can be divided into non-modifiable and modifiable factors (Van Dyke and Sheilesh, 2005). Examples of non-modifiable factors include aging, genetic predisposition, and osteoporosis; while modifiable factors include smoking, diabetes mellitus, psychological stress, alcohol consumption and poor oral hygiene (Van Dyke and Sheilesh, 2005; Borojevic, 2012; Reynolds, 2014; Hong et al., 2016; Wang and McCauley, 2016; Koo and Hong, 2018; Liu et al., 2018; Masumoto et al., 2019).
The role of the yet-identified parasitic fraction of the oral microbiome, namely: Entamoeba gingivalis and Trichomonas tenax, is gaining interest as potential contributing factors to the development of periodontal disease (Marty et al., 2017; Bonner et al., 2018; Eslahi et al., 2021). Several studies aimed to investigate oral colonization by these parasites among healthy individuals and those with periodontal disease with variable results (Athari et al., 2007; Ghabanchi et al., 2010; Al-hamiary et al., 2011; Trim et al., 2011; Ibrahim and Abbas, 2012; Bonner et al., 2014; Yazar et al., 2016; Hassan et al., 2019). Such variability can be related to adoption of different approaches for parasite detection, and the existence of previously unknown genetic variants of oral parasites (Garcia et al., 2018b; Santi-Rocca, 2020). In addition, variability in the prevalence of oral parasites in health and disease can be attributed to limitations of small sample sizes, and possible bias in selection of study subjects among others as reviewed recently by (Santi-Rocca, 2020).
The objective of the current study was to investigate the prevalence of E. gingivalis and T. tenax in health and periodontal disease. Also, we aimed to better define the place of gingivitis in the physiopathology of periodontal disease using parasite colonization. Finally, we aimed to identify the variables that might be associated with increased likelihood of harboring these oral parasites in health and disease.
Materials and Methods
Study Design
The prospective study with active enrolment of potential participants was carried out at Jordan University Hospital (JUH), Amman, Jordan from July to December 2019.
We sought to recruit study subjects from the following three categories: (1) Individuals with healthy gingiva (will be referred to as "healthy group" in the rest of manuscript). This healthy group was defined based on a healthy periodontium with no attachment loss, no bleeding upon probing (BOP) or minimal BOP (<10%), and no anatomical loss of periodontal structures, with absence of clinical signs of inflammation; (2) Individuals with gingivitis (herein, the term "gingivitis" will be applied to plaque‐induced gingivitis, rather than non‐dental‐biofilm induced forms of gingivitis); and (3) individuals with periodontitis.
The individuals with gingivitis and those with periodontitis -which represented the "disease group"- were recruited from Periodontics Outpatient Clinics at JUH using a convenience sampling approach, whereas the healthy controls were recruited by active approach of the JUH staff that included dentists, laboratory technicians, nurses and students at the University of Jordan.
Ethical Statement
This study was approved by the School of Medicine and the School of Graduate Studies, University of Jordan. Ethical approval was obtained from the Institutional Review Board (IRB) at JUH (Ref. No. 239/2019).
A written and signed informed consent was obtained from all individuals who agreed to participate in the study following full explanation of the study objectives and the procedure of obtaining the samples (Supplementary Material). In addition, the work was conducted according to the principles of good clinical practice that have their origin in the declaration of Helsinki and all individual data were treated with confidentiality.
Eligibility Criteria for Participation in the Study
Each healthy individual was included in the study if the following criteria were met altogether: 1) Healthy gingiva on periodontal examination; 2) Bleeding index (BI) of less than 10%; and 3) No previous history of periodontal diseases. The BI was calculated as follows: six representative teeth from all quadrants were chosen, and each tooth was gently probed with a University of North Carolina (UNC) periodontal probe (15 mm) at four sites (mesial, mid-buccal, mid-lingual, and distal). A dichotomous reading was used where bleeding is scored as present (given a score of 1) or absent (given a score of 0) and the number of sites where bleeding is present was recorded. The BI as a percentage was then defined through dividing the number of sites where bleeding was recorded by the total number of sites tested multiplied by 100. A controlled gentle probing force [well-tolerated by the patient (25 g)] was used (Newbrun, 1996).
The inclusion criteria for individuals with gingivitis and periodontitis were: 1) Diagnosis of the periodontal disease for the first time; and 2) No previous history of exposure to any kind of periodontal therapy (scaling or root planing).
The presence of one of the following criteria resulted in exclusion of the potential participant from the study: 1) Pregnancy; 2) Previous history of periodontal treatment; 3) Non‐dental biofilm-induced forms of gingivitis; 4) Presence of dental implants; 5) Recent use of antibiotics; or 6) Orthodontics treatment.
Assessment of the Possible Risk Factors for Periodontal Disease
Data from the study participants were collected using a paper-based questionnaire (Supplementary Material). The study participants' data included: age, gender, nationality, body mass index (BMI), monthly income, dental care level, history of smoking, history of alcohol consumption, history of diabetes mellitus (DM), family history of gingival disease and history of osteoporosis.
In addition, nine questions (adopted from the Perceived Stress Scale) to assess the stress-related factors, with each positive answer given a single point yielding a stress score that ranged from nil to nine (Nielsen et al., 2016). The study population was divided into two groups based on stress score as follows: "lower stress group" with a stress score of zero to 4, and "higher stress group" with a stress score of 5 to 9. The Cronbach's α value of 0.855 indicated an acceptable internal consistency for the proposed stress scale in this study.
Classification of the Study Subjects
The diagnosis of gingivitis and periodontitis was based on diagnostic guidelines that were set by the 2018 new classification scheme for periodontal and peri-implant diseases and conditions (Caton et al., 2018).
The detailed approach of evaluating the study subjects is provided in (Supplementary Material). Bleeding on probing and supragingival plaque presence (1) or absence (0) were evaluated in 4 sites of 6 teeth (details in Supplementary Material). The mean of the 24 sites gave the bleeding index (BI) and the plaque index (PI), expressed as percentages. In addition, the periodontal screening and recording (PSR) score was evaluated (details in Supplementary Material) (Caton et al., 2018).
The study subject was classified into the "healthy group" based on a BI < 10% and periodontal screening and recording (PSR) score of zero. The sample from each healthy participant was composed of a sub-gingival plaque along with saliva.
If the BI was ≥ 10% and ≤ 30%, then the participant was considered to have a "localized gingivitis". The study subjects with BI > 30% were considered to have a "generalized gingivitis".
Study subjects were classified into the "periodontitis group" after full periodontal examination if there was interdental clinical attachment loss detectable at two or more non-adjacent teeth, or buccal or oral clinical attachment loss (CAL) ≥ 2 mm with pocket depth > 3mm detectable at two or more teeth. Once the patient was diagnosed with periodontitis, staging and grading were done according to the recent classification of 2018 (Caton et al., 2018; Papapanou et al., 2018).
Specimen Collection
Salivary and dental plaque specimens were obtained from each study subject. Supra-gingival plaque was removed then the sample was taken from the deepest periodontal pocket. Dental plaque samples were collected using the UNC periodontal probe (15 mm). For participants with furcation involvement, the sample was taken using Naber's probe (Nordent manufacturing Inc., Illinois, the United States) from the furcation. For each participant, the salivary and dental plaque specimens were mixed together in a sterile tube that was stored at −20°C for DNA extraction and amplification.
DNA Purification and Amplification
Purification of DNA from the saliva/dental plaque specimens was done using QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) according to manufacturer's instructions (Supplementary Material).
For the detection of oral parasites by PCR, two sets of PCR primers were used. For E. gingivalis ST1, we used the same set of primers utilized by Bonner et al. with a minor modification of the reverse primer as follows: forward primer (5′-AGGAATGAACGGAACGTACA-3′) and reverse primer (5′-CCATTTCCTTCTTCTATTGTTTMAC-3′) with a product size of 203 bases in the 18S ribosomal RNA region (Bonner et al., 2014).
For T. tenax, we used the same set of primers utilized by Kikuta et al. as follows: PT3 forward primer (5′-AGTTCCATCGATGCCATTC-3′) and PT7 reverse primer (5′-GCATCTAAGGACTTAGACG-3′) with product size of 776 bases in 18S ribosomal RNA region (Kikuta et al., 1997).
The PCR mix comprised 5 μL of the DNA eluate, 5 μL of 5×FIREPol Master Mix (Solis BioDyne), 1 μL of each primer and 13 μL of DNase/RNase free water. The steps of PCR were as follows: Initial denaturation for 3.5 minutes at 94°C, 40 cycles of 1 minute at 94°C for denaturation, 1 minute at 60°C for primer annealing, 1 minute at 72°C for elongation, a final elongation step for 5 minutes at 72°C (Kikuta et al., 1997; Kucknoor et al., 2009; Bonner et al., 2014).
Proper positive and negative controls (patient samples with motile Entamoeba and Trichomonas that yielded the expected amplicon sizes as positive controls, and nuclease-free water as the negative control) for purification and PCR were used to ensure the quality of DNA extraction and PCR and to rule out contamination. The housekeeping gene actin beta (ACTB) with accession number (NG_007992) was used to assess PCR inhibition of the sample and to ensure the efficiency of the DNA purification procedure with the following primers: forward 5'-GTCCTGTGGCATCCACGAAA-3' and reverse 5'-AGTGAGGACCCTGGATGTGAC-3' and the PCR product size was 265 bases.
Statistical Analysis
Data generated from the study were edited using Microsoft Excel and uploaded to IBM SPSS Statistics 22.0 for Windows (Armonk, New York, the United States: IBM Corp). Two-sided Fisher's exact test (FET), Chi-squared test (χ2 test), Mann-Whitney U (M-W), Kruskal-Wallis (K-W) and linear-by-linear test for association (LBL) tests were used when appropriate and the statistical significance was considered for p ≤ 0.050.
To analyse the patterns associated with higher likelihood of having periodontal disease as a whole and per disease state (gingivitis and periodontitis), we conducted multinomial logistic regression analysis using variables that were classified into dichotomous outcomes. Confidence intervals of percentages (95% CI) were calculated using modified Wald method through GraphPad calculator available freely online through the following link: https://www.graphpad.com/quickcalcs/ConfInterval1.cfm
Sample size determination was based on calculations done via Epitools - Epidemiological Calculators available freely online (https://epitools.ausvet.com.au/). The minimum number of participants in each study group was determined at 53 based on the following parameters in "Sample size for a Case-control study": Expected proportion in controls=0.05, assumed odds ratio=5.00, confidence level=0.90, and power=0.80
Results
Characteristics of the Study Population
The total number of study participants who were eligible to be included in final analysis was 237, distributed as follows: healthy group (n=94, 39.7%), gingivitis group (n=53, 22.4%) and periodontitis group (n=90, 38.0%, Table 1).
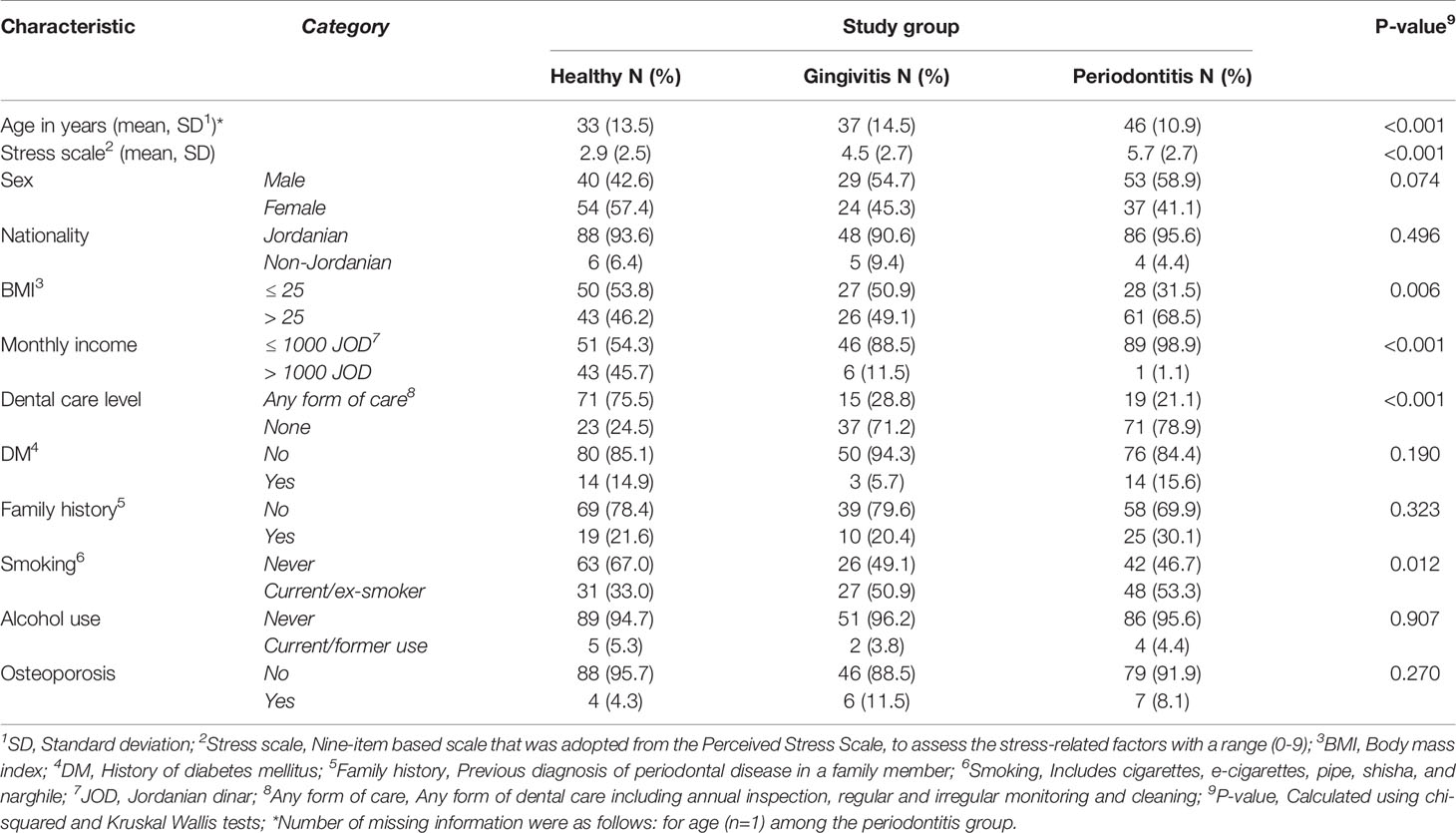
Table 1 Characteristics of the study population divided by the three study groups.
Significant differences among the three study groups were found for the following factors: the median age of the healthy group was younger compared to the disease group (24 vs. 44 years; p<0.001; M-W). The periodontitis group had an older median age compared to the two other groups (p<0.001; K-W, Table 1).
Additional differences between the three study groups were noticed as follows: higher BMI, lower monthly income, lack of dental care, and current/previous history of smoking were found for the periodontitis group (Table 1).
The Prevalence of Oral Parasites in the Whole Study Population
The PCR testing was performed for all study subjects (n=237). The overall prevalence of E. gingivalis among the study subjects was 71.7% (95% CI: 65.7% to 77.1%), while the overall prevalence for T. tenax was 12.2% (95% CI: 8.6% to 17.1%).
Stratified by the three study groups, the prevalence of E. gingivalis was the highest among the periodontitis group (n=80/90, 88.9%), compared to the gingivitis group (n=45/53, 84.9%), while the healthy group had the lowest prevalence (n=45/94, 47.9%, Figure 1). The difference in E. gingivalis prevalence was statistically significant upon comparing the healthy group to the gingivitis and periodontitis groups (p<0.001 for both comparisons; χ2 test). However, the difference was not statistically significant upon comparing the gingivitis group with the periodontitis group (p=0.603; χ2 test).
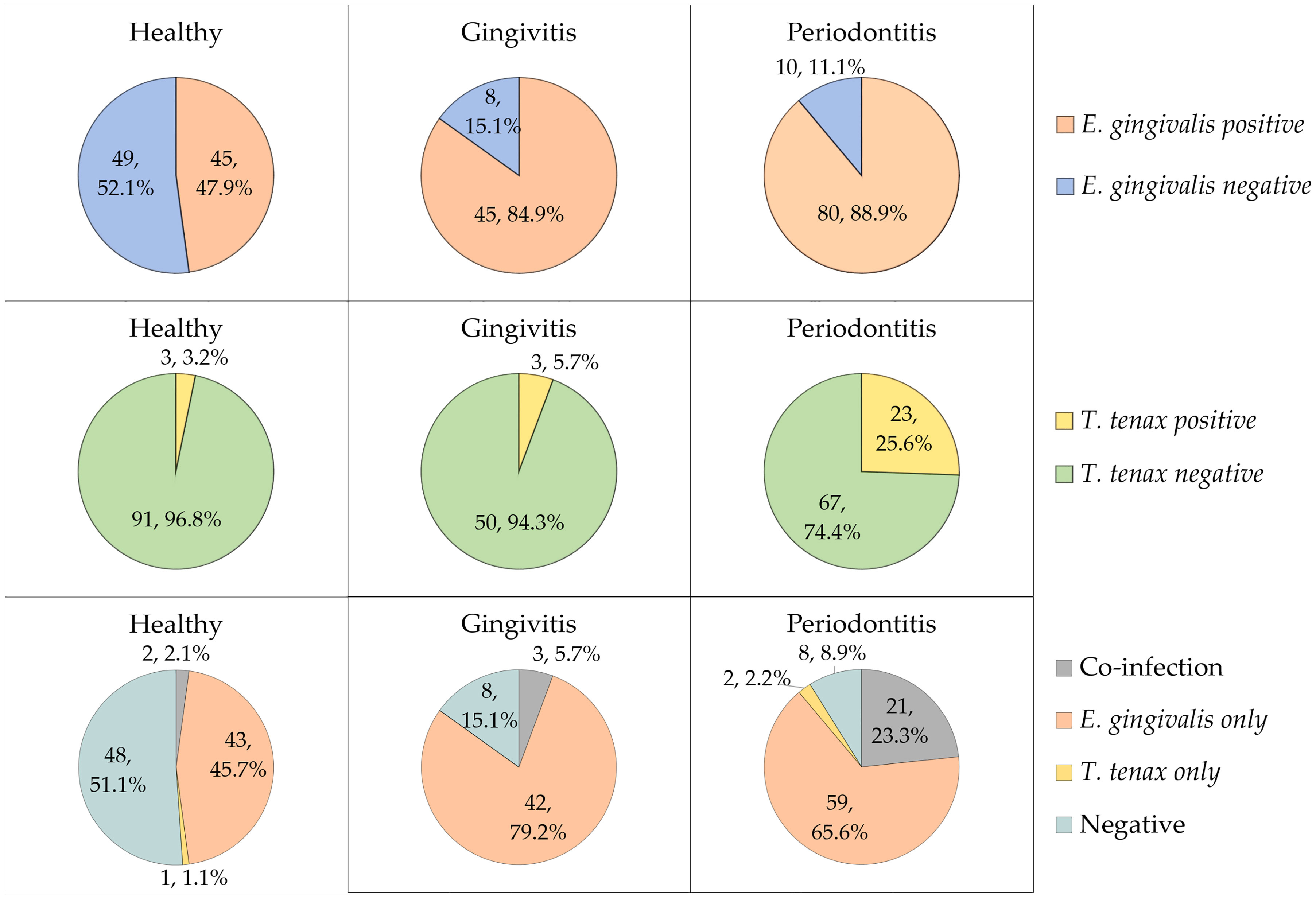
Figure 1 The prevalence of Entamoeba gingivalis and Trichomonas tenax in the study participants divided by study groups (health, gingivitis and periodontitis). Co-infection denoted the concurrent detection of E. gingivalis and T. tenax.
For T. tenax, the prevalence increased starting from 3.2% in the heathy group, to 5.7% in the gingivitis group and reaching 25.6% in the periodontitis group (Figure 1). The difference lacked statistical significance upon comparing the healthy and gingivitis groups (p=0.668; χ2 test). However, a statistically significant result was noticed upon comparing the periodontitis group to healthy and gingivitis groups (p<0.001 and p=0.003 respectively for the two comparisons; χ2 test).
Concurrent detection of the two oral parasites (dual colonization) was found in 26 study subjects yielding a prevalence of 11.0% (95% CI: 7.6% to 15.6%). Among the 29 study subjects with T. tenax colonization, E. gingivalis was also detected in 26 individuals (89.7%).
Factors Associated With a Higher Prevalence of Oral Parasites in Each Study Group
We aimed to seek the possible factors associated with a higher prevalence of E. gingivalis. However, as some parameters from these populations were impacted by our recruitment, as previously cited, we chose to perform this analysis without merging the three study groups. A higher prevalence of E. gingivalis in the healthy group was found among participants with a history of DM compared to those who did not have the disease (78.6% vs. 42.5%; p=0.019, FET, Table 2). For the other tested variables, the prevalence of E. gingivalis in each study group did not show statistically significant differences (Table 2).
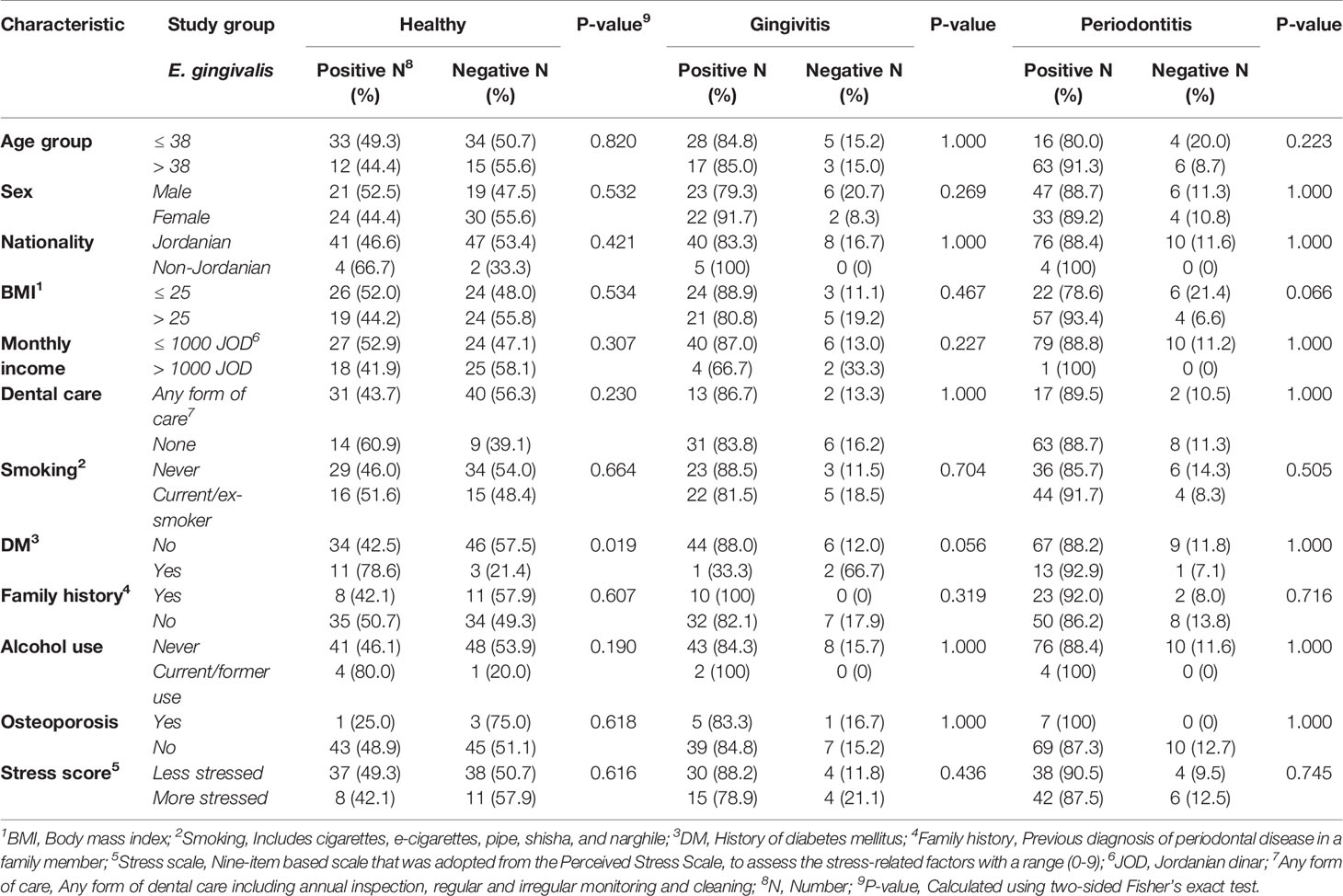
Table 2 Factors associated with a higher prevalence of Entamoeba gingivalis stratified per study group.
For T. tenax, a higher prevalence was found in the periodontitis group among non-Jordanians (100.0% vs. 22.1%; p=0.003, FET), and among periodontitis patients with a BMI > 25 (32.8% vs. 10.7%; p=0.036, FET). In the gingivitis group, participants with a higher level of stress had a higher prevalence of T. tenax (15.8%) compared to its total absence among the less stressed participants in this group (p=0.041, FET). For the other tested variables, a lack of statistically significant differences in the prevalence of T. tenax within each study group (healthy, gingivitis and periodontitis) was noticed (Table 3).
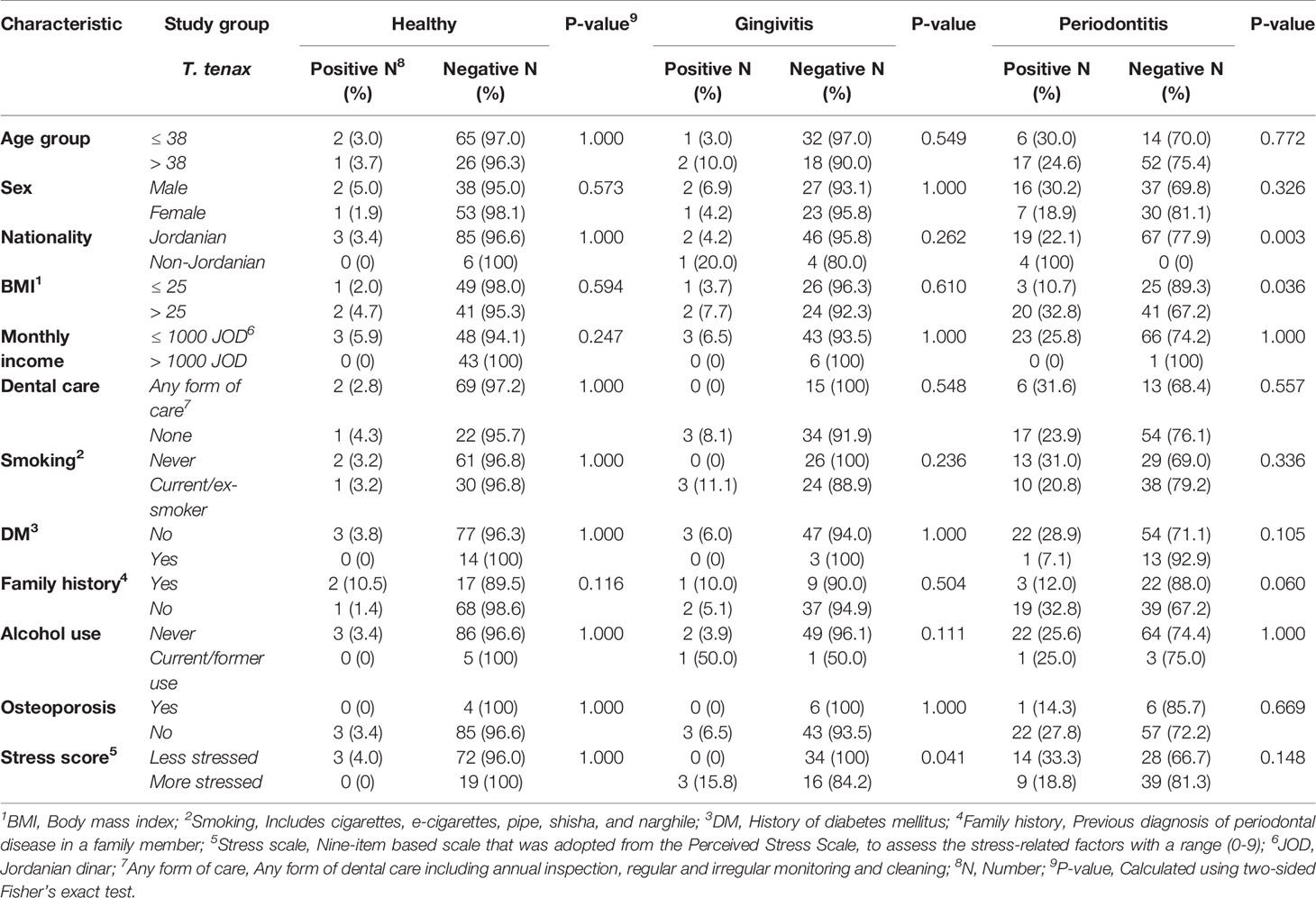
Table 3 Factors associated with a higher prevalence of Trichomonas tenax stratified per study group.
Risk Factors for Periodontal Disease
The majority of risk factors for periodontal disease that were previously reported in various studies were tested in this work (e.g. dental care level, smoking, DM, etc.). To analyse the patterns associated with higher likelihood of having periodontal disease as a whole and per disease state (gingivitis and periodontitis), we conducted multinomial logistic regression analysis using the following variables as covariates that were classified into dichotomous outcomes as follows: age [> 38 years vs. ≤ 38 years, (38 years was the median age for the whole population)], sex (male vs. female), nationality (Jordanian vs. non-Jordanian), BMI (> 25 vs. ≤ 25), monthly income (≤ 1000 JOD vs. > 1000 JOD), dental care (no dental care vs. any form of dental care), smoking (current/ex-smoker vs. non-smoker), DM vs. non-diabetic, family history (present vs. absent), subjective evaluation of stress (more stressed if the score is 0-4 vs. less stressed if the score is 5-9), alcohol use (current/ex-user vs. non-consumer), osteoporosis (present vs. absent).
Initial analysis was done with the dependent variable being health vs. periodontal disease and the presence/absence of oral parasites as the fixed factors. Positivity for E. gingivalis was correlated with the presence of periodontal disease with odds ratio (OR) of 6.6 (95% CI: 2.7 – 16.5; p<0.001), with the following covariates having significant correlation with the disease: lower monthly income (OR: 8.2, 95% CI: 2.6 – 25.8, p<0.001), the lack of dental care (OR: 4.8, 95% CI: 2.1 – 11.1, p<0.001), and history of DM (OR: 4.5, 95% CI: 1.3 – 15.8, p=0.017). Oral colonization by T. tenax was not found to be correlated with the presence of periodontal disease (p=0.180, Figure 2A).
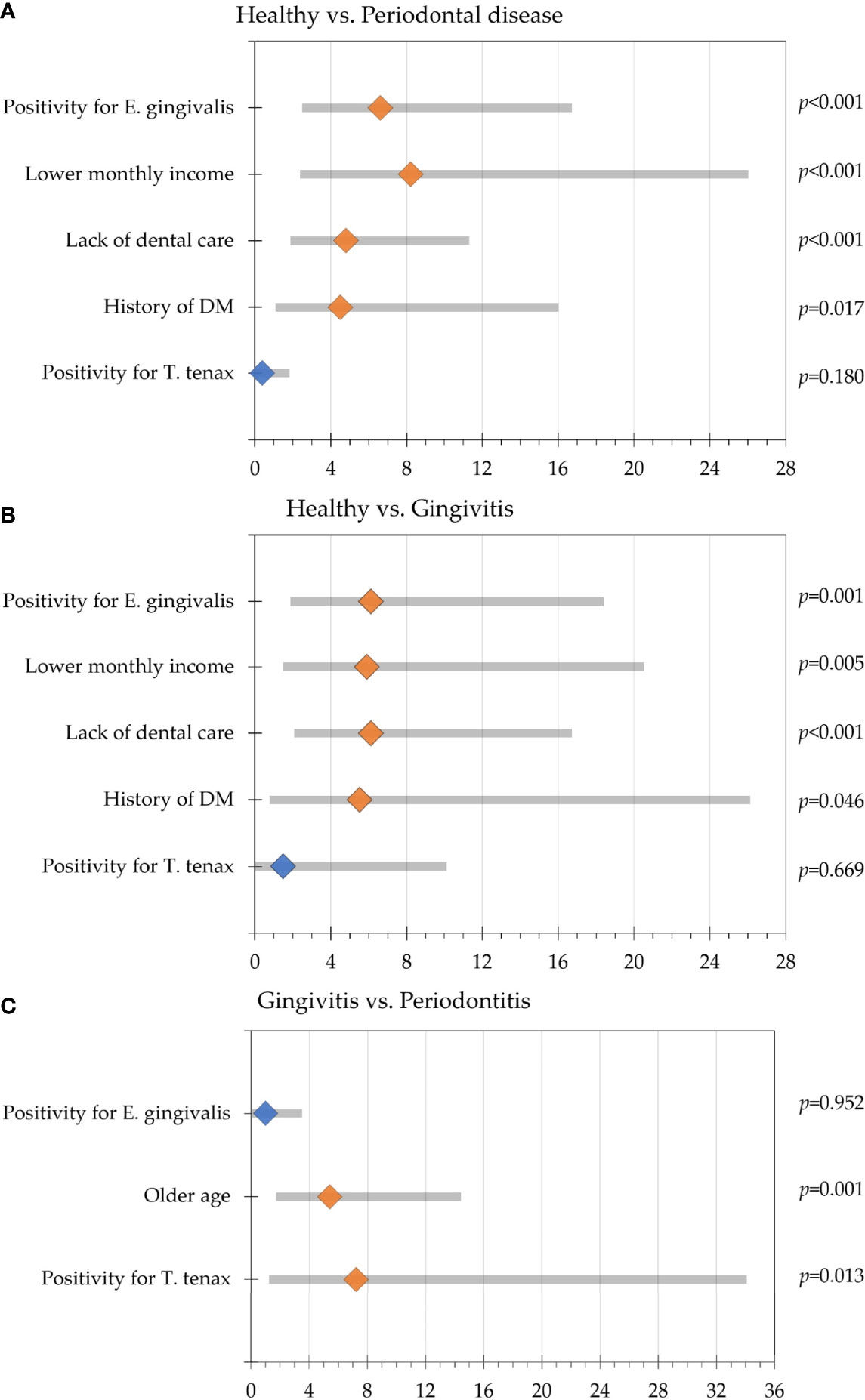
Figure 2 Multinomial regression analysis of participants' variable association with periodontal disease. Odds ratios are represented by the diamond shapes (light orange for statistically significant result and blue color for statistically non-significant results), while the 95% confidence intervals are shown as the grey bars. (A) Comparison between the healthy group and the periodontal disease group (gingivitis and periodontitis); (B) Comparison between the healthy group and gingivitis group; (C) Comparison between the gingivitis and periodontitis groups.
Further analysis with dependent variable being health vs. gingivitis and the presence/absence of oral parasites as the fixed factors revealed that colonization by E. gingivalis was correlated with gingivitis (OR: 6.1, 95% CI: 2.1 – 18.2, p=0.001), while correlation with oral colonization by T. tenax lacked the statistical significance for upon comparing the healthy group with gingivitis (p=0.669). The covariates that were associated with gingivitis in relation to the healthy group were the lack of dental care (OR: 6.1, 95% CI: 2.3 – 16.5, p<0.001), lower monthly income (OR: 5.9, 95% CI: 1.7 – 20.3, p=0.005) and history of DM (OR: 5.5, 95% CI: 1.0 – 29.5, p=0.046, Figure 2B).
Comparison between the healthy and the periodontitis groups revealed that colonization by E. gingivalis and T. tenax were significantly correlated with periodontitis (OR: 6.4, 95% CI: 2.2 – 18.7, p=0.001 for E. gingivalis, and OR: 4.7, 95% CI: 1.0 – 21.8, p=0.045 for T. tenax). The covariates that were associated with periodontitis in relation to the healthy group were the lack of dental care (OR: 4.4, 95% CI: 1.6 – 11.7, p=0.003), lower monthly income (OR: 26.6, 95% CI: 2.8 – 249.7, p=0.004) and history of DM (OR: 4.9, 95% CI: 1.3 – 19.3, p=0.021).
Comparing the gingivitis and periodontitis groups revealed that T. tenax was significantly correlated with periodontitis (OR: 7.2, 95% CI: 1.5 – 33.8, p=0.013), while colonization by E. gingivalis lacked the statistical significance upon comparing the two groups (p=0.952). Besides colonization by T. tenax, older age was the only covariate to be correlated with periodontitis compared to gingivitis (OR: 5.4, 95% CI: 2.0 – 14.2, p=0.001, Figure 2C).
Association of Oral Parasites With Periodontal Disease Extent
Data on the type of periodontal disease (localized vs. generalized) was available from 130 study subjects. The localized type comprised 18 individuals as opposed to 112 individuals with generalized periodontal disease. T. tenax was only detected among individuals with generalized periodontal disease compared to its total absence among those with localized periodontal disease (19.6% vs. 0.0%; p=0.039, χ2 test) and no dual colonization was detected either in the localized group compared to generalized group (p=0.051, χ2 test). Despite the higher prevalence of both oral parasites in individuals with advanced stage and grade of disease (as indicated by higher BI and PI), the differences lacked statistical significance as illustrated in (Figure 3).
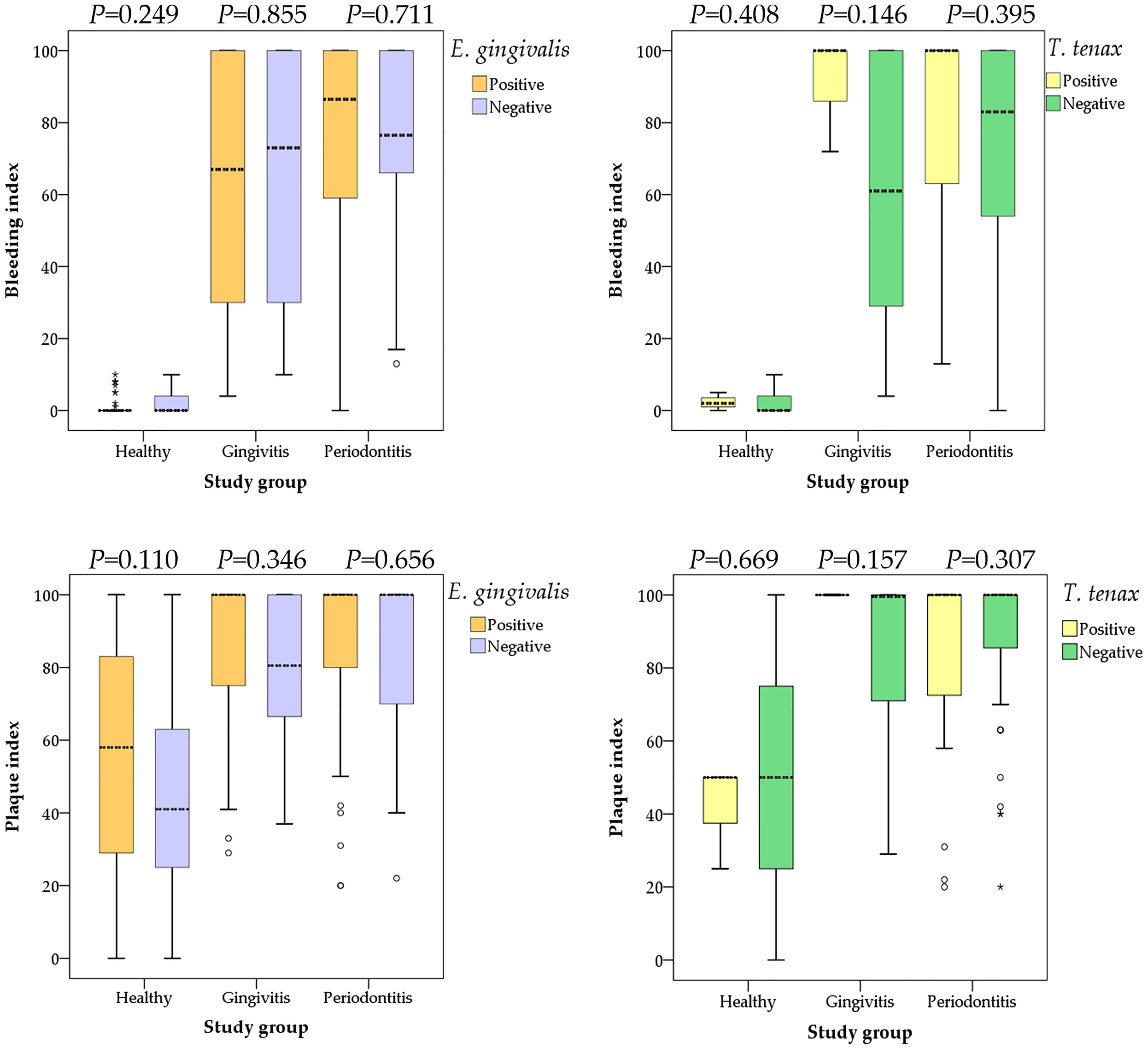
Figure 3 Comparison between the bleeding and plaque indices with colonization by oral parasites stratified by the study groups (health, gingivitis and periodontitis). P values were calculated using Mann Whitney U test. Median values are shown as dashed lines. Outlier values are shown as small circles, while extreme outlier values are shown as asterisks.
Estimation of the Co-Infection Rates by the Two Oral Parasites in the Study Sample
The concurrent detection of the two oral parasites was observed in 26 study subjects yielding a co-infection rate of 11.0% (95% CI: 7.6% – 15.6%). The co-infection rates were higher among periodontitis patients, individuals older than 38 years, non-Jordanians, participants with BMI > 25, participants with monthly income ≤ 1000 JOD, and those who reported the lack of any form of dental care (Table 4).Multinomial logistic regression analysis was conducted, with study group as the dependent variable, oral parasite status (co-infection vs. E. gingivalis only vs. T. tenax only vs. negative) as the fixed factor and the following covariates: age (> 38 years vs. ≤ 38 years, [38 years was the median age for the whole population]), sex (male vs. female), nationality (Jordanian vs. non-Jordanian), BMI (> 25 vs. ≤ 25), monthly income (≤ 1000 JOD vs. > 1000 JOD), dental care (no dental care vs. any form of dental care), smoking (current/ex-smoker vs. non-smoker), DM vs. non-diabetic, family history (present vs. absent), subjective evaluation of stress (more stressed if the score is 0-4 vs. less stressed if the score is 5-9), alcohol use (current/ex-user vs. non-consumer), osteoporosis (present vs. absent). Analysis showed the odds of periodontitis compared to the healthy group was the highest among individuals with coinfection (OR: 32.3, 95% CI: 4.3 – 236.2, p=0.001), followed by E. gingivalis only (OR: 6.5, 95% CI: 2.1 – 20.7, p=0.001), whereas the sole presence of T. tenax or negative result did not yield a statistically significant result (Table 5). On the other hand, co-infection by the two oral parasites did not yield significant correlations between the healthy vs. gingivitis groups or between gingivitis vs. periodontitis groups (Table 5).
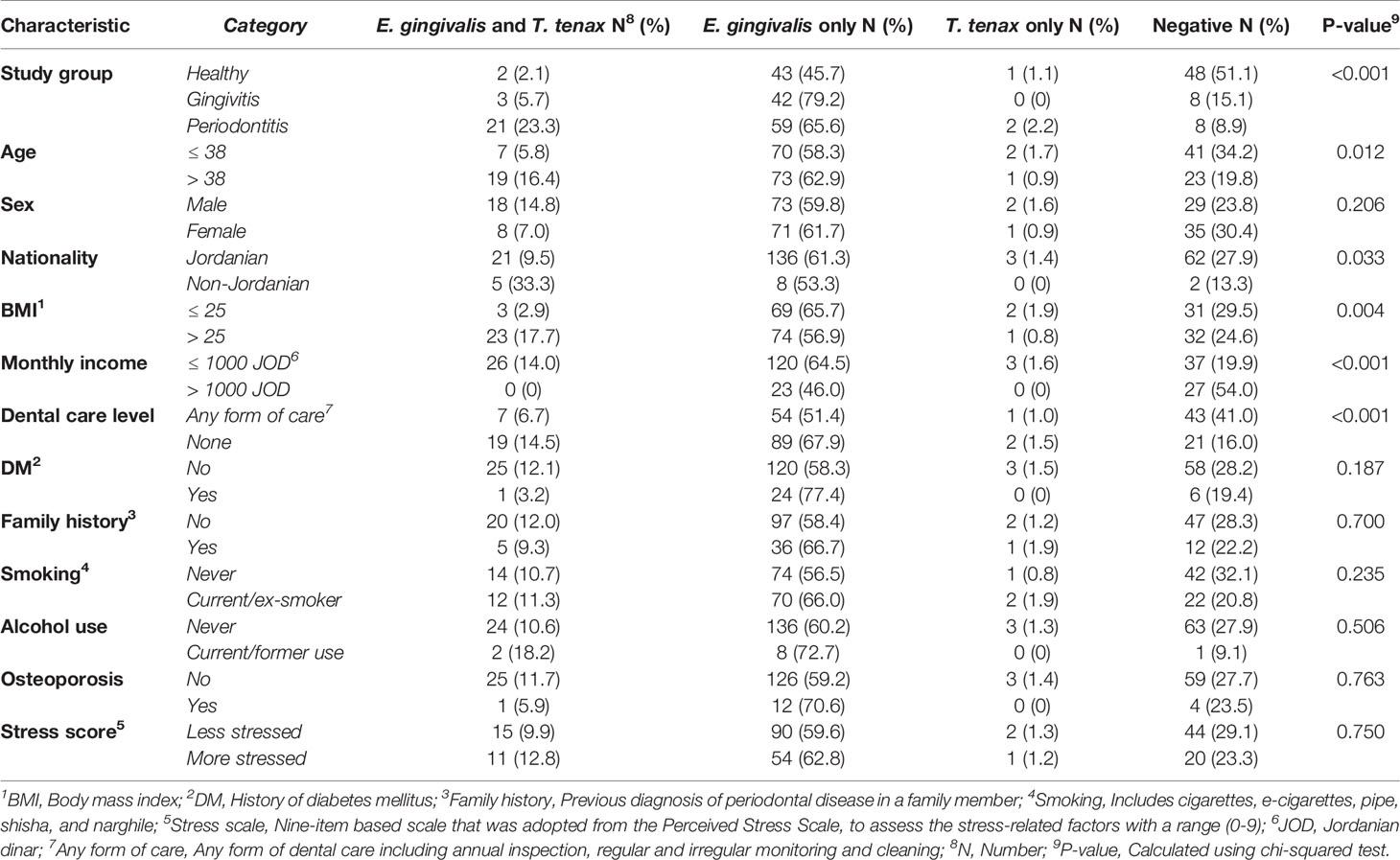
Table 4 Factors associated co-infection by Entamoeba gingivalis and Trichomonas tenax in the study sample.
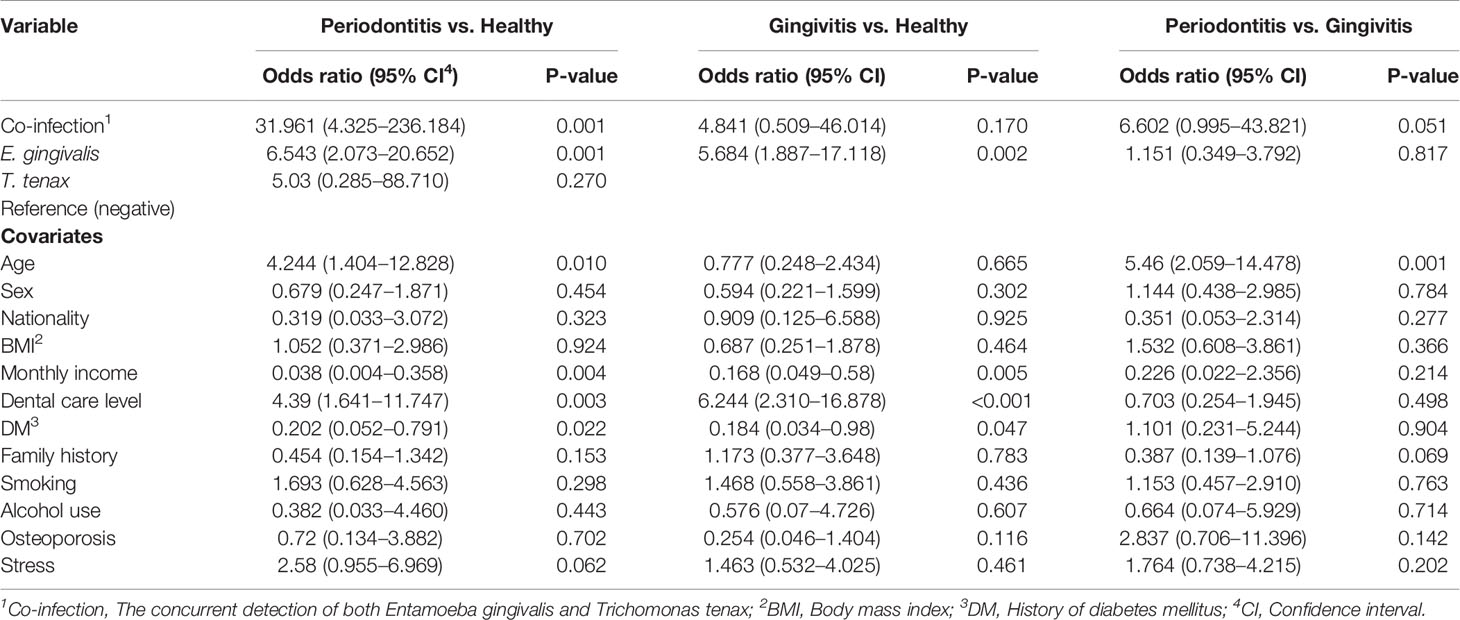
Table 5 Multinomial regression analysis assessing the correlation between oral parasite detection and disease states (healthy vs. gingivitis vs. periodontitis).
Comparison of the BI and the PI among the three study groups (healthy vs. gingivitis vs. periodontitis) based on the oral parasite infection status (co-infection vs. E. gingivalis only vs. T. tenax only vs. negative result) did not yield any statistically significant differences (Figure 4).
Figure 4 Comparison between the bleeding and plaque indices with oral parasite infection status stratified by the study groups (health, gingivitis and periodontitis). Co-infection denoted the concurrent detection of both E. gingivalis and T. tenax. P values were calculated using Kruskal Wallis test. Median values are shown as dashed lines. Outlier values are shown as small circles, while extreme outlier values are shown as asterisks.
Discussion
Gingivitis and periodontitis are inflammatory conditions that can also be viewed as infectious diseases (Cekici et al., 2014). In periodontitis, the role of the bacterial fraction of the oral microbiome has been studied extensively with accumulating evidence pointing to its contribution to the etiology of the disease (Teles et al., 2013). However, the fraction of protozoa was not studied to a similar level compared to its bacterial counterpart (Deng et al., 2017; Bonner et al., 2018; Bisson et al., 2019; Santi-Rocca, 2020; Badri et al., 2021; Eslahi et al., 2021). Nevertheless, this protozoan fraction does not seem to be negligible in some clinical setups, with E. gingivalis RNA accounting for up to 9% of the total RNA in periodontal pockets (Deng et al., 2017). Thus, more studies are warranted to evaluate the potential role of the human oral "protozoome" in health and disease.
In the current study, we investigated the prevalence and risk factors for oral colonization by the currently known human oral parasites, E. gingivalis and T. tenax, for the first time in Jordan. The importance of this work is related to the following aspects: First, periodontal disease (gingivitis and periodontitis) has a high prevalence among individuals of different age groups, which poses significant risks to public health, including various associated conditions and potential tooth loss (Griffin et al., 2012; Kinane et al., 2017; Tonetti et al., 2017). Second, some key elements regarding the etiology and pathophysiology of periodontal disease have not been disentangled yet; hence, more research is needed to decipher these unresolved elements (Darveau, 2010; Hajishengallis, 2015; Lamont et al., 2018). Third, despite the uncertainties regarding the specific roles of different microorganisms in periodontal disease, the accumulating evidence points to conspicuous differences in the oral microbiome between health and disease, and the role of oral parasites in both states has not been clearly delineated yet (Santi-Rocca, 2020). Fourth, a few studies on the epidemiology of oral parasites originated from the Middle East and North Africa (MENA) region. A majority of these studies that were conducted in Egypt, Iran, Iraq and Saudi Arabia, have not relied on the molecular detection approach for estimating the burden of oral parasites (el Hayawan and Bayoumy, 1992; Athari et al., 2007; Ghabanchi et al., 2010; Ibrahim and Abbas, 2012; Ismail et al., 2017; Hassan et al., 2019).
Thus, we aimed to build on the previous work that had the same objective, while attempting to avoid some limitations of the previously published reports. For example, in this study, each sample was based on a mixture of saliva and dental plaques, as used for instance by (Bao et al., 2020), as opposed to relying on one of them solely for parasite detection, as used for instance by (Bonner et al., 2014). The advantage of this sampling approach is related to improving the sensitivity of detection as oral parasites can be found in either one of these sites (Rashidi Maybodi et al., 2016). Also, we relied on the reference molecular method currently applied for oral parasite detection, rather than relying on microscopic detection (Bonner et al., 2014; Santi-Rocca, 2020). In addition, we tried to improve the sensitivity of molecular detection in this study through using silica column-based DNA extraction method, which can help to remove PCR inhibitors, and we adopted sensitive PCR protocols that were previously validated (Kikuta et al., 1997; Schrader et al., 2012; Bonner et al., 2014).
The main result of the study was the finding of a profoundly higher prevalence of E. gingivalis (87.4%) among individuals with periodontal disease, compared to those in the healthy group (47.9%) using the PCR method. For T. tenax, the estimates were much lower and significant differences were found moving from 3.2% in the healthy group to 18.2% among individuals with periodontal disease. To assess the reproducibility of these results, a limited number of studies were found (Kikuta et al., 1996; Trim et al., 2011; Bonner et al., 2014; Garcia et al., 2018a; Santi-Rocca, 2020; Badri et al., 2021; Eslahi et al., 2021). The comparisons were further complicated by the reliance of a majority of the previous studies on microscopic detection methods (examination of wet mounts or permanent stained smears) (Athari et al., 2007; Ghabanchi et al., 2010; Al-hamiary et al., 2011; Ibrahim and Abbas, 2012; Yazar et al., 2016; Hassan et al., 2019). Possible explanations for discrepancy between the results of microscopic detection of oral parasites and the PCR-based results include: the subjectivity of the microscopic approach which depends on the skills and experience of the examiner, the number of the fields examined, the method of microscopy used (light vs. phase-contrast), the use of staining, the nature of mounting media, and the lag time between sampling and examination (particularly for wet mount examination which depends on the viability of oral parasites, since motility is one of the decisive defining features for diagnosis) as discussed previously by (Bonner et al., 2014).
Despite the variability in results of the previously published reports, two recurring patterns were observed and were in line with our results. First, the observation of an increase in the prevalence of both oral parasites moving from health to gingivitis and reaching the highest levels in periodontitis (Santi-Rocca, 2020; Badri et al., 2021). Second, the generally higher prevalence of E. gingivalis in comparison to T. tenax in both health and disease. Interestingly, a significant association between the presence of T. tenax and periodontal disease severity was also found which was manifested in its total absence in localized disease. This result should be interpreted with extreme c...
Comments
Post a Comment