Creep in nitroimidazole inhibitory concentration among the ... - Nature.com
Abstract
Infections by Entamoeba histolytica (E. histolytica) lead to considerable morbidity and mortality worldwide and treatment is reliant on a single class of drugs, nitroimidazoles. Treatment failures and intermittent reports of relapse from different parts of world indicate towards development of clinical drug resistance. In the present study, susceptibility testing of clinical isolates of E. histolytica was carried against metronidazole and tinidazole. Additionally, anti-amoebic property of active compounds of Andrographis paniculata was also evaluated. Prevalence of metronidazole resistance gene (nim) in patients attending hospital was also done to get comprehensive insight of present situation of drug resistance in E. histolytica. Mean inhibitory concentration 50 (IC50) value of E. histolytica isolates against metronidazole and tinidazole was 20.01 and 16.1 µM respectively. Andrographolide showed minimum mean IC50 value (3.06 µM). Significant percentage inhibition of E. histolytica isolates by andrographolide was seen as compared to metronidazole (p = 0.0495). None of E. histolytica isolates showed presence of nim gene. However, in stool samples from hospital attending population, prevalence of nimE gene was found to be 76.6% (69/90) and 62.2% (56/90) in diarrheal and non-diarrheal samples respectively. Inhibitory concentration of commonly used nitroimidazoles against clinical isolates of E. histolytica are on rise. Percentage inhibition of E. histolytica isolates by andrographolide was significantly higher than control drug metronidazole.
Introduction
The choice of drugs for the treatment of infections by Entamoeba usually depends on the diagnosis and the severity of the disease. The World Health Organization (WHO) recommends that all patients infected with Entamoeba histolytica (E. histolytica) should be treated1. However, owing to the unavailability and the associated toxicity, treatment of infections due to E. histolytica is reliant on a single class of drugs, nitroimidazoles, for more than half a century2. The safety and efficacy of the nitroimidazoles in all forms of amoebiasis has been firmly established3. The most used agent from this class is metronidazole. Metronidazole has been the front-line choice for a number of anaerobic and protozoan infections worldwide.
However, indiscriminate overuse, over-the-counter (OTC) sales and inappropriate treatment regimens has resulted in the increase in the minimum inhibitory concentration (MIC) of the drug4. Appearance of the metronidazole resistant strains in-vivo and in-vitro can be an indication of the emerging drug resistance. However, the studies about the metronidazole resistance and the associated mechanisms from India are very limited5. Detection of resistance genes from the clinical samples without pure isolates can be helpful in epidemiological studies on the distribution of the resistance genes. Additionally, the antimicrobial susceptibility testing through culture methods provides information of the resistance genes of only micro-organisms which are capable of growing under given conditions.
Cases of treatment failures even after adequate therapy from different parts of the world indicate development of clinical drug resistance. Reports of recurrence in the cases of extraintestinal manifestation of E. histolytica are on rise6,7,8,9. There are reports of multiple relapse and treatment failures despite following appropriate management6. Nonetheless, resistance to metronidazole, although reported in Giardia lamblia, Trichomonas vaginalis and Leishmania donovani is still not documented in the case of E. histolytica isolates10,11,12,13.
Ten nitroimidazole resistance genes (nim A to nim J) have been identified till date that confer reduced sensitivity to 5-nitroimidazole drugs14,15,16. The proposed mechanism for the resistance by nim gene states that they encode an enzyme called, 5-nitroimidazole reductase, that inhibit the formation of the nitroso radicals critical for the antimicrobial activity17. It has been demonstrated that the high level of metronidazole resistance can be easily induced in strains containing nim gene15. The increased frequency of the nim gene even after short exposure to metronidazole treatment has been reported.
Since last 60 years, no new drug has developed against E. histolytica infections, despite the urgencies set by National Institute of Allergy and Infectious diseases (NIAID) for drug development for treatment of category B pathogens. In-vitro drug resistance in metronidazole is not a serious problem presently, however, the increasing inhibitory concentrations and cases of treatment failures is heralding towards the clinical resistance6,7,8. Additionally, there are several adverse effects associated with the long-term use of metronidazole including diarrhoea, loss of appetite, metallic flavour, induction of genotoxic effects, DNA damage as well as oxidative cell damage3,18,19. The most critical reactions include effect on central nervous system, peripheral neuropathy, ataxia, vertigo, convulsions and cerebellar toxicity20,21,22. Thus, it becomes important to reconsider the risk benefit relation and individual's susceptibility in case of metronidazole prescription.
The revaluation of different medicinal plants for their therapeutic properties has gained much importance due to the emerging drug resistance to the commonly used agents. One such plant species is Andrographis paniculata (A. paniculata). A. paniculata is a medicinal herb with numerous pharmacological properties. The abundant therapeutic use of this plant has been demonstrated in the tropical and sub-tropical regions of South-east Asia23. These regions are also endemic for the Entamoeba infections leading to considerable morbidity and mortality24,25,26. Andrographolide is the sole major compound isolated from A. paniculata, which has been studied for numerous medicinal properties. Neo-andrographolide and andrograpanin are the other dominant and important diterpenoids isolated from the aerial part of A. paniculata27. In our experience, significant anthelmintic property, and the quantitative estimation of the marker compounds in the extracts of leaves of A. paniculata has been recently reported against human hookworm isolates28.
In our tertiary care center, we have previously studied high prevalence of E. histolytica in amoebic liver abscess (ALA) and high recurrence of ALA in a 2 year follow up study8,29. The location of the present study is on the Ganga basin which has been associated with higher incidence of ALA in an endemic neighbouring country. The unusual rate of recurrence could hint towards the gradual development of the clinical resistance towards the commonly used drugs in this geographical area. Consequently, we hypothesized whether clinical resistance with metronidazole treatment is reflected in drug susceptibility testing of E. histolytica. With this background, the objective of this study was to evaluate the drug susceptibility of the clinical isolates of E. histolytica causing ALA against metronidazole and tinidazole. Along with it, the analysis of the anti-amoebic activity of active compounds of A. paniculata (andrographolide, neo-andrographolide and andrograpanin) was done. Additionally owing to the dearth in existing data, the prevalence of metronidazole resistance gene (nim gene) in stool samples of patients attending hospital was also carried out, to provide a comprehensive picture of the current situation of drug resistance in E. histolytica.
Results
Confirmation of the samples and demographic details
All the 15 liver aspirates included in the study were found to be positive for E. histolytica through nested multipex polymerase chain reaction (PCR) for Entamoeba sp. However, only 2 (13.3%) stool samples of these ALA patients were positive for E. histolytica. As we have previously reported increased recurrence rate in ALA patients thus, the isolation of the trophozoites were carried out from the liver aspirate samples. Figure 1 shows the presence of the trophozoites in the liver aspirate samples. Among the diarrheal samples included in the present study 3 (3.3%) samples were positive for E. histolytica.

Microscopic image of the trophozoites in the liver aspirate samples (40x).
The mean age of the ALA patients was 41.06 ± 7.3 years. Majority (13/15, 86.6%) of the participants were males. Considering their residence, 9 (60%) participants were from rural background and 6 (40%) were from urban areas. Out of the total, 5 (33.3%) participants had received higher education (above class 5th), 7 (46.6%) participants had primary education and 3 (20%) were uneducated. Majority (11/15, 73.3%) participants were employed. Five (33.3%) participants were from low and 10 (66.6%) participants were from middle economic status. In the present study, the incidence of ALA was more frequent in middle-aged males with rural background. However, no significant correlation was found between the demographic factors and the incidence of the ALA owing to the inclusion of only the diseased ALA cases (no controls) and the small sample size of the study.
Drug susceptibility testing of E. histolytica clinical isolates
The mean IC50 value of the E. histolytica isolates against the metronidazole and tinidazole was found to be 20.01 µM and 16.1 µM respectively. The mean IC50 values were significantly higher (p = 0.022) for metronidazole as compared to the tinidazole. Figure 2 shows the graphical representation of the mean percentage inhibition of the 15 clinical isolates of E. histolytica by the metronidazole and tinidazole. As it is evident from the Fig. 2 that the inhibition percentage of the tinidazole was higher than the metronidazole against the clinical isolates of E. histolytica throughout the concentration range included in the study. A list of studies showing details of the drug susceptibility testing of the E. histolytica isolates using various assays has been shown in Table 1.
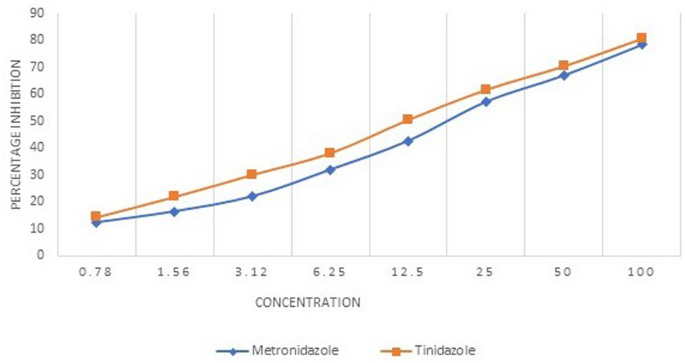
Percentage inhibition of E. histolytica clinical isolates by metronidazole and tinidazole.
Anti-amoebic property of active compounds of A. paniculata
The mean IC50 value of the E. histolytica isolates against the active compounds and the control drug, metronidazole has been shown in Table 2. Andrographolide showed the minimum mean IC50 value (3.06 µM) against the E. histolytica clinical isolates. The graphical representation of the comparison of mean percentage inhibition of the 15 clinical isolates of E. histolytica by the active compounds and the drugs, metronidazole and tinidazole has been shown in Fig. 3. Among the active compounds andrographolide showed the maximum percentage inhibition followed by neo-andrographolide and andrograpanin. All the three active compounds showed better percentage inhibition in comparison to the commonly used nitroimidazoles. The activities of the active compounds as compared to each other was not found to be significant. However, there was a significant percentage inhibition of the E. histolytica isolates by andrographolide as compared to the metronidazole (p = 0.0495).
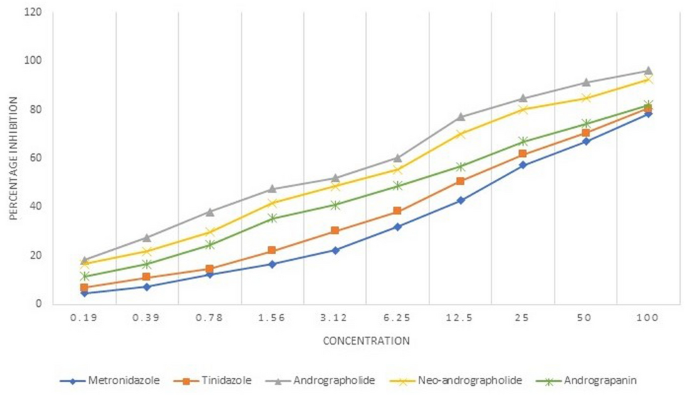
Percentage inhibition of E. histolytica clinical isolates by andrographolide, neo-andrographolide and andrograpanin in comparison to the metronidazole and tinidazole.
Prevalence of nim genes
None of the E. histolytica isolates showed the presence of nim gene. In the case of stool samples, the prevalence of nim gene in diarrheal and non-diarrheal stool samples was found to be 76.6% (69/90) and 62.2% (56/90) respectively. The prevalence of nim gene was significantly more in the diarrheal samples (p = 0.036). Among the three E. histolytica positive diarrheal samples 2 (66.6%) samples showed the presence of nim gene and out of 87 E. histolytica negative diarrheal samples 67 (77%) showed the presence of nim gene. No significant correlation between the presence of nim gene in the E. histolytica positive and negative diarrheal samples was found (p = 0.68).
Through analysis of the digested fragments from both the enzymes, all the samples positive for nim gene were found to be nim E type. The fragments obtained by digestion from Hin1II and Taq1 restriction enzyme has been shown in Fig. 4. The restriction digestion products obtained by Hin1II enzyme showed a single fragment of 441 bp and Taq1 enzyme produced two fragments of 274 bp and 155 bp on agarose gel electrophoresis.
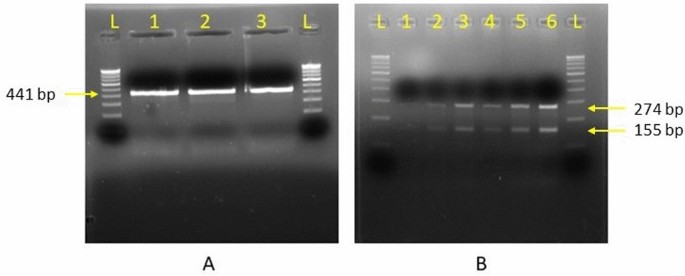
Restriction digestion products obtained by (A) Hin1II enzyme; 441 bp and (B) Taq1 enzyme; 274 bp and 155 bp.
The sequence analysis of the nim gene PCR products confirmed the presence of nim E gene type. A sequence homology of 99% with the nim E gene type was revealed through Basic local alignment search tool (BLAST) analysis from the available database. (Accession number: AM117602.1, https://www.ncbi.nlm.nih.gov/nuccore/AM117602.1/).
Bacterial flora in the diarrheal samples
Among the microflora associated with diarrheal samples Prevotella (34, 37.7%) was predominant followed by Bacteroides (20, 22.2%), Escherichia coli (16, 17.7%), Klebsiella (12, 13.3%) and C. freundii (8, 8.8%). Majority (54, 78.2%) of the diarrheal samples showing the presence of nim gene showed the presence of either Prevotella or Bacteroides.
Discussion
Drug susceptibility testing in E. histolytica has been challenging because of the absence of competent screening assay. Additionally, the available methods are exhaustive and have complex procedures besides requiring expertise and parasite culture facilities30. Thus, only a handful of studies are available reporting the drug sensitivity of E. histolytica isolates.
The most interesting point was that though the findings of the present study were in agreement with the previous reports, yet the mean IC50 values in the present study were higher than the prior studies10,31. A study conducted 18 years before in India on the drug sensitivity of the E. histolytica isolates had shown the mean IC50 value of 13.2 µM and 12.4 µM for metronidazole and tinidazole respectively10. The increase in the mean IC50 values can be attributed to the emergence of clinical resistance or due to the differences in the culture techniques of the parasite, strains involved or raw materials used. Studies have reported the minimum MIC of 12.5–25 µM for the laboratory passaged E. histolytica strains. Another study described mean IC50 value of 18.47 µM and a cut off value of > 30 µM for resistance in the E. histolytica isolates32. However, majority of the studies on the drug sensitivity of E. histolytica are outdated and there is a dearth of recent data against the commonly used anti-amoebic agents. Additionally, the comparison of the available studies regarding the drug sensitivity of E. histolytica particularly becomes difficult because of the use of the different methods for the measurement of the activity of the drugs.
Various studies have reported the sensitivity of the drugs against the E. histolytica isolates in terms of minimum lethal concentration (MLC), MIC, IC50, effective dose 50 (ED50) etc32,33,34,35. However, it has been suggested to report the sensitivity of a drug as molar concentration (µM) to standardize the comparison of efficacy of the drugs especially in cases when metronidazole is compared with other nitroimidazoles with significantly higher molecular weight31. The clinical isolates included in the present study were maintained in the monoxenic culture as it has been previously reported that presence of bacterial flora along with the amoeba did not significantly interfere with the performance of the test or the sensitivity values10,36.
The percentage inhibition of the E. histolytica clinical isolates by marker active compound andrographolide was significantly higher than the control drug metronidazole. Reports have suggested that andrographolide and its analogues possess some novel mechanism of action responsible for their effects37. A recent study using andrographolide based nanoparticles has shown that they generate intracellular reactive oxygen species that promote damage to the vital biomolecules including DNA, proteins and lipids and cause membrane leaking leading to the release of the cytoplasmic components and death38.
In the malarial parasite, andrographolide had shown inhibitory activity towards the ring-stage of the parasite affecting the protein and nucleic acid synthesis. Andrographolide has been designated as the 'Transcription blocker' in the parasite Plasmodium falciparum39. In the andrographolide treated filarial parasite the activity of antioxidant enzymes and glutathione-S-transferase (GSH) has been found to be reduced in concentration dependent manner40. Additionally, in parasites treated with andrographolide an increase in the activity of NADPH oxidase has been seen which leads to the development of hydrogen peroxide (H2O2) and other hazardous reactive oxygen species splitting the mitochondrial membrane organization40. Even though andrographolide has been found to be effective as a traditional medicine in dysentery, a detailed study on the exact mode of action of andrographolide in E. histolytica is lacking.
In the present study, the nim gene was not detected in any of the clinical isolates of E. histolytica. However, the present hospital-based study revealed high level prevalence of the nim gene in the diarrheal as well as non-diarrheal stool samples of the patients. In other anaerobic micro-organisms, such as clinical isolates of Bacteroides spp. and Prevotella spp. the carriage rate of 0–2.8% and 0–8% respectively has been reported17,41,42,43. There is no data on the presence of nim genes in E. histolytica clinical isolates for commenting on this study finding. The nim gene associated metronidazole resistance has been known to vary among the diverse geographical regions44. In the present study the decreased susceptibility of the clinical isolates of E. histolytica towards commonly used nitroimidazoles does not seem to be associated with the nim genes. Previous studies have suggested that the reduction in sensitivity to metronidazole in the E. histolytica isolates can be because of several factors, some of which are non-enzymatic45. The increased expression of the iron super dismutase (Fe-SOD) and peroxiredoxin along with the decreased expression of ferredoxin1 and TrxR constitutes important component involved in the mechanism of metronidazole resistance in E. histolytica46.
High prevalence of nim gene in stool samples of the patients attending the tertiary care centre is in concordance with another study from India that showed high copy number of the nim gene in the stool samples of the patients with gastrointestinal discomfort as well as in the healthy individual from the community47. Such high prevalence of the nim gene in this geographical region can be attributed to the OTC sales of the nitroimidazoles (especially metronidazole), which has been linked with the increased frequency of the nim gene induction48. Also, the presence of nim gene was found to be significantly associated with the diarrheal stool samples (p = 0.036). This could be due to the significant abundance of the nim gene carrying micro-organisms such as Bacteroides spp. and Prevotella spp. in the diarrheal stool samples49,50.
The presence of nim E gene type in all the positive samples has been reported in the present study. Our finding is in-line with the previous studies reported from India which described nim E to be the most common nim gene type circulating in this geographical region5,47,51. In contrast to our results, studies from other part of the world have shown nim A gene followed by nim B and nim D gene to be most prevalent type14,15. However, no possible correlation between the type of nim gene and the degree of resistance has been ever described in the literature.
The scope of the present work was to analyse the susceptibility pattern of the clinical isolates of E. histolytica towards the commonly used drugs. Along with it, the anti-amoebic property of the marker compounds of A. paniculata was studied. To the best of our knowledge, this is the first report of the anti-amoebic activity of the active compounds from the extracts of leaves of A. paniculata against the clinical isolates of E. histolytica. A detailed study of the toxicity profile, dose determination and the delivery options of the active compounds is required to establish them as a potent anti-amoebic agent in the future.
The study was not without any limitation as only 15 clinical isolates of E. histolytica were included in the study owing to the difficulty in the establishment and maintenance of the parasitic culture. The drug susceptibility assays could not be carried out in the axenic culture due to lack of the culture and maintenance facility. Additionally, the drug susceptibility assay was performed using only two drugs, metronidazole and tinidazole. However, these drugs are the main line of treatment for the E. histolytica infections including ALA cases and therefore provides important insight into the situation.
Material and methods
Ethics statement
The study was ethically approved by Institute Ethical Committee, Faculty of Medicine, Institute of Medical Sciences, Banaras Hindu University (Dean/2016–17/EC/045). All experiments in the present study were performed in accordance with guidelines and regulations provided by the Institute Ethical Committee. The study included only adults and written informed consent was obtained from all the subjects who participated after explaining them the purpose of the study.
Inclusion/exclusion criteria
Patients with well-defined liver abscesses greater than 5 cm diameter which were confirmed through abdominal ultrasound were included in the present study. Patients who have gone through aspiration previously or were on any medication from past four weeks were excluded from the study. Furthermore, the liver aspirate showing presence of associated bacteria either by aerobic culture or by molecular detection through 16-S rRNA primers were excluded from the study52.
Clinical samples and isolates of E. histolytica
Fifteen isolates from 15 E. histolytica positive ALA samples from patients attending the Gastroenterology and Radiology department of Sir Sunderlal Hospital, Varanasi, India were included in this study. As the trophozoites are majorly attached to the wall of the abscess, the use of a series of smaller bottles for the collection of the liver aspirate was done to keep the last fraction undiluted by the main mass of material53.
The stool samples from these ALA patients were also collected. A pre-tested questionnaire about the demographic details including age, gender, residence, education, employment and economic status of the patients were collected through experienced research scholars.
Additionally, diarrheal (n = 90) and non-diarrheal (n = 90) stool samples from patients attending different outpatient departments of Sir Sundarlal Hospital, Varanasi were also included to detect the frequency of the nim genes in this geographical region. All the diarrheal samples were inoculated on the Mac Conkey agar medium and blood agar medium for overnight incubation at 37 °C. Additionally screening of common anaerobes from previous literature was done in the diarrheal samples using conventional PCR29.
Confirmation of the samples
Direct microscopy was performed for all the samples through wet mount to screen for the presence of trophozoites of Entamoeba spp. Nested multiplex PCR was performed for the molecular detection of E. histolytica in all the samples using species specific primers targeting 16S like rRNA gene54. The reaction mixture preparation and reaction conditions were as described previously29.
Isolation and culture of the trophozoites
Trophozoites were isolated from liver aspirate of ALA patients by culture in modified Boeck and Drbohlav's monoxenic medium with few modifications53,55. Locke-egg medium was prepared. For this, Locke's solution was prepared by dissolving the 8.0 g sodium chloride (Sigma-Aldrich Chemicals Pvt. Ltd, India), 0.2 g calcium chloride (Sigma-Aldrich Chemicals Pvt. Ltd, India), 0.2 g potassium chloride (Sigma-Aldrich Chemicals Pvt. Ltd, India), 0.01 magnesium chloride (Sigma-Aldrich Chemicals Pvt. Ltd, India), 2.0 g sodium phosphate (Sigma-Aldrich Chemicals Pvt. Ltd, India), 0.4 g sodium bicarbonate (Sigma-Aldrich Chemicals Pvt. Ltd, India) and 0.3 g potassium phosphate, monobasic (Sigma-Aldrich Chemicals Pvt. Ltd, India) into 1L distilled water. The Locke solution was autoclaved at 15 min at 121 °C under a pressure of 15 lb/in2. Any precipitate formed was removed by filtration by Whatman no. 1 paper.
For egg slant preparation, fresh hens' eggs were sterilized by flaming in 70% ethanol and broken into a graduated cylinder. Locke's solution (12.5 ml) per 45 ml of egg was added and then emulsified in a Waring-type blender and filtered through gauze into a flask. A total of 5 ml of the emulsified egg were added to standard culture tubes (16 by 125 mm) and sterilized by inspissation. After cooling of the slants, they were overlayed with 6 ml of Locke's solution.
Deactivated bovine serum (Biological industries, Kibbutz Beit-Haemek, Israel) and sterilized rice starch (VWR International, Poole, United Kingdom) were added to the medium before addition of the samples56. Along with it, a loopful of pure colonies of Klebsiella pneumoniae which was susceptible to the metronidazole was inoculated to the culture tubes before the addition of the clinical sample. The sub-culturing was performed every 48 h and the trophozoites were further subjected to drug susceptibility testing mostly after two passages.
Chemicals and antimicrobial agents
The pure salts of drug, metronidazole and tinidazole were included in the study (Sigma-Aldrich Chemicals Pvt. Ltd, India) The concentration range of 0.78 µM—100 µM were used. Andrographolide (CAS No. 5508–58–7, purity > 95%), neoandrographolide (CAS.No. 27215–14–1, purity > 95%) and andrograpanin (CAS No. 82209–74–3, purity > 95%) were obtained from Natural Remedies Pvt. Ltd (Bangalore, India) and used in the concentration of 0.19 µM—100 µM.
Drug susceptibility testing (DST)
For the susceptibility testing, Robinson medium for Entamoeba twin pack (HiMedia laboratories Pvt. Ltd, Mumbai, India) was used. Susceptibility testing to the standard drugs and the active compounds was performed as described previously10. Briefly, the trophozoites were harvested from 24 h old culture from the interface of the locke's solution and the egg slant and the count were adjusted to 1 × 105 trophozoites per ml. The in-vitro susceptibility testing was performed in 96 wells microtiter plates. The doubling dilutions of the drugs and active compounds was performed to get the required concentrations. Diluted trophozoite suspension was added and the plates were incubated for 4 h at 37 °C. Following incubation, the plate's content was discarded and the plate was washed. Further, 100 µl of nitroblue tetrazolium (HiMedia laboratories Pvt. Ltd, Mumbai, India) was added to each well and the plates were incubated at 37 °C for 45 min. After incubation, the plates were again washed and DMSO (200 µl/well) was added. Thereafter the plates were incubated at 37 °C for 10 min. Following incubation, the optical density (OD) was measured. Each isolate was tested against the drugs and active compounds in duplicates. Each test included a control well containing only media and the trophozoites without drugs and a blank well containing only media. The measurement of OD in each test was done at 540 nm using microtiter plate reader (LisaScan® EM Elisa plate reader, Transasia Bio-Medicals Ltd, India). The percentage of non-viable trophozoites at each concentration was calculated using the formula:
The mean IC50 values for all the clinical isolates against the drugs (metronidazole, tinidazole) and the active compounds (andrographolide, neoandrographolide and andrograpanin) was calculated using "Quest Graph™ IC50 Calculator" (AAT Bioquest, Inc.)
Screening of the nim gene
The 15 E. histolytica isolates and stool samples from the diarrheal and non-diarrheal patients were screened for the presence of nim genes. DNA extraction was carried out from all the isolates and the stool samples by using QIAGEN stool mini kit (Qiagen, Germany) as per the manufacturer's instructions.
The detection of nim genes in the E. histolytica isolates and stool samples was carried out by conventional PCR using the universal set of primers, Nim-3 and Nim-5, for all known nim genes57.
Determination of nim gene type
The determintion of the nim gene type was done by restriction fragment length polymorphism (RFLP). The PCR products obtained in the previous step were digested by using two restriction enzymes Taq1 (New England Biolabs, Massachusetts, United States) and Hin1II (New England Biolabs, Massachusetts, United States) as per the manufacturer's instructions15,58. For nim gene type confirmation, PCR products were subjected to DNA sequencing of the partial region of SSU rRNA gene.
Statistical analysis
The mean IC50 value of the clinical isolates of E. histolytica were compared by Student's t-test using 2019 MedCalc software (version:bv). The presence of the nim gene in diarrheal and non-diarrheal stool samples was also compared using 2019 MedCalc software (version:bv). In the case of the active compounds, metronidazole was used as the control drug. A p value < 0.05 was considered to be significant.
Data availability
All the data generated or analysed during the study has been included in the manuscript.
References
Center for Disease control and prevention. https://www.cdc.gov/parasites/amebiasis/general-info (2019).
Shirley, D.A., Farr, L., Watanabe, K. & Moonah, S. (2018) A review of the global burden, new diagnostics, and current therapeutics for amebiasis. In: Open forum infectious diseases, Oxford University Press, United Sates.
Gonzales, M., Leonila, F. & Juliet, S. A. Antiamoebic drugs for treating amoebic colitis. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD006085 (2019).
Martínez-Palomo, A. & Martínez-Baez, M. Selective primary health care: Strategies for control of disease in the developing world. X. Amebiasis. Rev. Infect. Dis. 5, 1093–1102 (1983).
Sethi, S. et al. Emerging metronidazole resistance in Bacteroides spp. and its association with the nim gene: A study from North India. J. Global Antimicrob. Resist. 16, 210–214 (2019).
Ramiro, M. et al. Reincidence of amebic liver abscess: A case report. Arch. Med. Res. 31, S1-3 (2000).
Hwang, E. W., Cheung, L., Mojtahed, A. & Cartwright, C. A. Relapse of intestinal and hepatic amebiasis after treatment. Dig. Dis. Sci. 56, 677–680 (2011).
Singh, A., Banerjee, T. & Shukla, S. K. Factors associated with high rates of recurrence of amebic liver abscess (ALA) in North India. Am. J. Trop. Med. Hyg. 104, 1383–1387 (2021).
Alam, F. et al. Amebic liver abscess in northern region of Bangladesh: Sociodemographic determinants and clinical outcomes. BMC Res. Notes. 7, 1–5 (2014).
Bansal, D., Sehgal, R., Chawla, Y., Mahajan, R. C. & Malla, N. In vitro activity of antiamoebic drugs against clinical isolates of Entamoeba histolytica and Entamoeba dispar. Ann. Clin. Microbiol. Antimicrob. 3, 1–5 (2004).
Voolmann, T. & Boreham, P. Metronidazole resistant Trichomonas vaginalis in Brisbane. Med. J. Aust. 159, 490–490 (1993).
Sundar, S. et al. Failure of pentavalent antimony in visceral leishmaniasis in India: Report from the center of the Indian epidemic. Clin. Infect. Dis. 31, 1104–1107 (2000).
Nash, T. E. et al. Treatment of patients with refractory giardiasis. Clin. Infect. Dis. 33, 22–28 (2001).
Gal, M. & Brazier, J. S. Metronidazole resistance in Bacteroides spp. carrying nim genes and the selection of slow-growing metronidazole-resistant mutants. J. Antimicrob. Chemother. 54, 109–116 (2004).
Lofmark, S., Fang, H., Hedberg, M. & Edlund, C. Inducible metronidazole resistance and nim genes in clinical Bacteroides fragilis group isolates. Antimicrob. Agent. Chemother. 49, 1253–1256 (2005).
Alauzet, C. et al. Metronidazole resistance in Prevotella spp. and description of a new nim gene in Prevotella baroniae. Antimicrob. Agents Chemother. 54, 60–64 (2010).
Husain, F. et al. Two multidrug-resistant clinical isolates of Bacteroides fragilis carry a novel metronidazole resistance nim gene (nimJ). Antimicrob. Agents Chemother. 57, 3767–3774 (2013).
Comments
Post a Comment