Dynamic changes of viral load and the duration of viral shedding in patients with hand, foot and mouth disease: a protocol for longitudinal study - BMC Infectious Diseases - BMC Infectious Diseases
Study design
This is a hospital-based prospective cohort study, and study methodological design will follow the requirements of 'Strengthening the Reporting of Observational Studies in Epidemiology' (STROBE) checklist for cohort studies [17]. During February 1 to December 31, 2022, we will seek to enroll and trace hospitalized HFMD patients in a designated referral hospital. The demographic and clinical data of HFMD patients during the whole clinical course and series clinical specimens will be collected to evaluate the dynamic changes of viral load and the duration of viral shedding.
Study site
The study will be conducted at the public health clinical center of Chengdu, capital city of Sichuan Province in Western China. It is a designated hospital for the diagnosis and treatment of HFMD in Sichuan Province, as well as a referral hospital for critically ill cases. In addition to general enterovirus testing, all hospitalized patients tested for EV-A71, CV-A16, CV-A6 and CV-A10 serotypes to confirm diagnosis since 2018. There were on average 1500 hospitalized HFMD cases annually before 2019, which provides sample size guarantee for the smooth development of the project.
Study subjects
All hospitalized patients meeting the following inclusion and exclusion criteria during February 1 to December 31, 2022 will be recruited in this study. The initial minimum sample size of this study is expected to reach about 120–160 cases. If the sample does not meet the minimum required follow-up volume, we will appropriately extend the project time.
Inclusion criteria
- (1)
Admitted to public clinical center of Chengdu due to HFMD induced by CV-A6, CV-A10 and CV-A16.
- (2)
Written informed consent from a parent or legal guardian for/and children.
Exclusion criteria
- (1)
Discharge from hospital within 24 h of admission.
- (2)
Patients from the suburbs of each district/county in the third circle of Chengdu and refuse to go to the hospital for follow-up.
Sample size calculation
The sample size for this study is 120 -160 within a period of 1 year, based on the inclusion rate of HFMD hospitalized patients in previous longitudinal studies [18], the number of hospitalized patients with HFMD in 2020, and a 20 percent loss to follow-up. In addition, according to previous studies, the multi-level model could guarantee relatively accurate parameter estimations when the high level sample is not less than 50 [19, 20].
Study procedure
Study subjects enrolled for this study need to conduct the procedures as below. Figure 1 provides an overview of the study procedure.
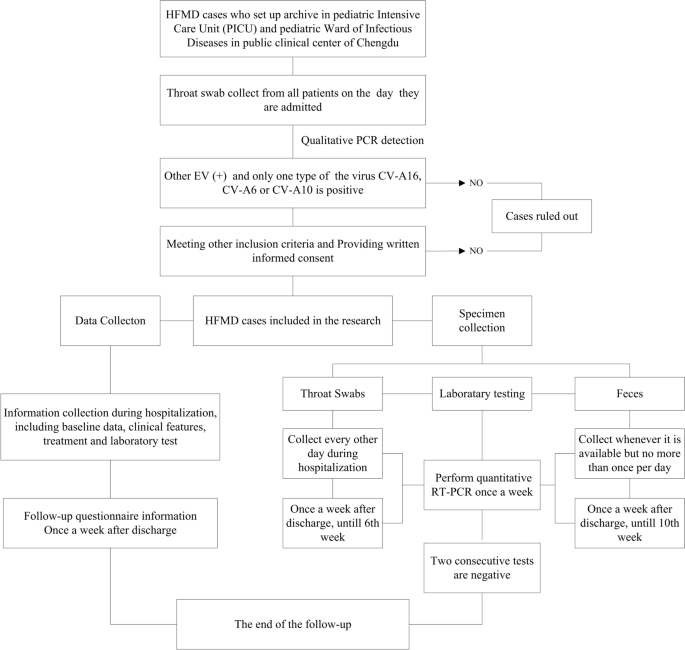
The follow chart of this study
Enrollment of subjects
The throat swabs of all HFMD cases will be collected on the day they are admitted to clinical public center of Chengdu to conduct qualitative PCR detection to determine virus types. Every patients singly induced by CV-A6, CV-A10 or CV-A16 will be approached after laboratory determined as soon as possible. All patients providing with written informed consent from their parent or legal guardian and/or themselves will be included in the study. We will uniformly code all patients who voluntarily participate in this project.
Clinical data collection
A structured hospitalization questionnaire including four parts will be used to collect detailed clinical data, which spans the length of a typical course of illness. In the first part, baseline data including demographics, medical history, birth history, symptoms and signs at the onset, treatment before admission and history of EV-A71 vaccination will be collected through interview of the patients and their parents. In the second part, clinical data including detailed clinical features and complications will be collected through ward rounds, and updated every day during hospitalization. The last two parts are clinical laboratory tests and treatments, which will be exported and collected from the electronic medical record system after patients discharge from the hospital. The data collection will begin as soon as possible after the cases enter the study.
After discharge, a structured follow-up questionnaire is used to investigate the patient's health status since the last follow-up, including the occurrence of suspected HFMD symptoms, medical history, diagnosis results, and the occurrence of other HFMD cases in the same family.
Clinical specimen collection
During hospitalization, throat swabs will be collected once every other day, while feces will be collected whenever it is available but no more than once per day as long as the volume meets the requirement. After discharge, we will conduct a home follow-up to collect throat swabs and feces once a week until two consecutive test results are negative, or reach the end of follow-up time (6th week after admission for throat swabs and 10th week for feces). All specimens will be collected according to the operating standards of the National Center for Disease Control and Prevention. All throat swabs and stool will be coded and stored in the 4 °C refrigerator temporarily (no more than 24 h) before transferred to − 80 °C freezer.
Laboratory test
Viral RNA extraction
The preprocessing of all the clinical specimens and extraction of viral nucleic acid will perform in Level 2 Biosafety laboratory (BSL-2). For throat swabs samples, Mix the virus sampling tube on a vortex shaker for at least 10 s to wash off the adhering virus or virus-containing cells, the mixture will be taken directly for RNA extraction. For stool samples, a stool suspension will be made by adding a bean size feces in to 700μL normal PBS buffer and mechanically oscillate for 30 s to make the sample fully mixed. The suspension will be then centrifuged at 12,000 rmp for 5 min, and the upper suspension will be used for RNA extraction. ABT Viral RNA extraction Kit (ABT, Beijing, China) will be used for RNA extraction in the development of the assay and RNA extraction from clinical specimens according to the manufacturer's instructions. The extracted viral RNAs will be immediately used for real-time fluorescence quantitative PCR detection. If not, the RNAs will be eluted with 40 µl of diethyl pyro carbonate-treated water and kept at − 80 °C until further use for real-time RT-PCR.
Real-time fluorescence quantitative PCR
The viral load of CV-A6, CV-A10 and CV-A16 will be detected by real-time fluorescence quantitative reverse transcription polymerase chain reaction (RT-PCR). The RT-PCR kit is produced by ABT Biotechnology Company (Beijing, China), the experimental operation will be carried out according to the manufacturer's instructions. The instrument is BIO-RAD CFX96 real-time fluorescence quantitative PCR instrument.
Outcome measures
Primary outcome
The primary outcome of the study is the dynamic changes of viral load and the duration of viral shedding in HFMD patients under the influence of related factors. To obtain this result, we will first identify the positive rate and virus load of the specific serotype enterovirus at each collection time points and the time of turning negative of HFMD patients. Then, we will use Bayesian multilevel model to fit the influence of nested fixed and random factors on the variation of viral load.
Secondary outcomes
The secondary endpoints include number of days spent in hospital, the proportion of clinical manifestation and their duration, as well as the epidemic features of hospitalized HFMD patients induced by CV-A6, CV-A10 or CV-A16.
Statistical analysis
We will use epidata3.1 to enter the data and R4.0.1 to conduct statistical analysis. We will follow the "Reporting of studies Conducted using 'Observational Routinely Collected Health Data' Statement" [21]. Our primary analysis will be the dynamic changes of viral load and the duration of viral shedding in HFMD patients, while clinical features and epidemic features of hospitalized HFMD cases will be secondary analysis.
Baseline characteristics
For continuous variables, we will summarize them using means ± standard deviation and the comparison between the two groups will use independent sample t-test if the data conform the normal distribution. If not, we will describe interquartile ranges, and nonparametric test will be used to compare the two groups. For categorical variables, we will report data with proportion (%) and compare groups through standard Pearson's, or Fisher's exact test if either group contains less than 10 patients. or Fisher's exact test if either group contains less than 10 patients. The level of significance will be set at p < 0.05.
Bayesian multilevel model
Bayesian multilevel model will be used to reflect the dynamic changes of viral load and the duration of viral shedding in HFMD patients under the influence of related factors. Because of the structural nesting and unbalanced characteristics of the research data, we will use Bayesian multilevel model to fit the influence of nested fixed and random factors on the variation of viral load.
Data management and publication
Initial study data collection is expected to be completed in late 2022. De-identified data is expected to be available a month after that. All data will be collected through paper questionnaires and entered manually by the research team. The database will be stored in an encrypted manner with Epidata3.1 limited to authorized personnel only, and the paper questionnaire will be sealed in the project office. Scientific publications or reports will not identify individual participants.
Results of the study will be submitted for publication in international peer reviewed biomedical journals. Position of authorship will be determined according to international standards for authorship in peer-reviewed journals.
Study status
All implementation site and laboratory preparations have been completed. Pre-investigation is currently conducting and will be finished on January 31, 2022, and the samples collected in pre-investigation will be used to laboratory operation process training. Formal investigation and participant recruitment will be carried out from February 1 to December 31, 2022.
Comments
Post a Comment